The \alpha,\beta$-unsaturated molecular orbital diagram is constructed from the ethene and carbonyl molecular orbitals
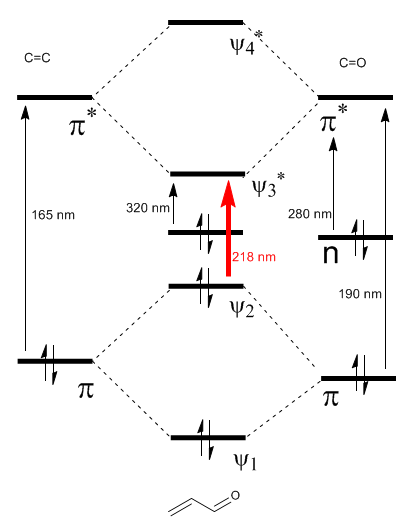
As can be seen in the diagram, the lowest energy transition allowed for the carbonyl occurs at 190 nm. However, the conjugation with the double bond produces two new molecular orbitals $\psi_2$ and $\psi_{3}^{\ast}$ whose energy difference is smaller, leading to a transition to a higher wavelength, $ λmax=218nm.
Note that transitions from the free pair are not considered to be prohibited.