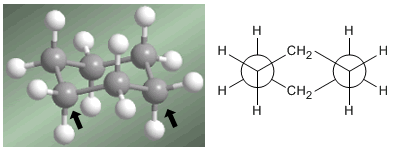
Professor Odd Hassel of the University of Oslo established that the most stable conformation of cyclohexane is the chair form. With bond angles of 111º the chair is almost free of angular tension. Also, all the links are staggered as can be seen in the Newman projection.
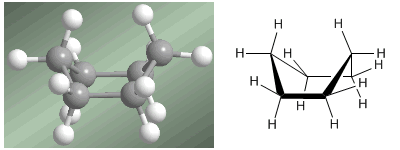
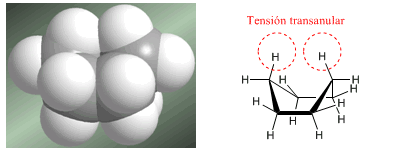
The second, much less stable conformation of cyclohexane is the boat . In this conformation, the angles are close to the tetrahedral arrangement and the angular strain is minimal. However, the boat is destabilized by the repulsion between the hydrogens that are located towards the interior of the boat. This repulsion is called transannular tension. This tension can be seen in the following models.
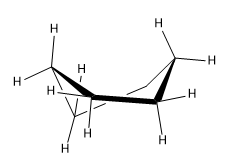
Twist form of cyclohexane
Avoid transannular tension by twisting the boat to remove the opposing hydrogens from the same plane.
Avoid transannular tension by twisting the boat to remove the opposing hydrogens from the same plane.
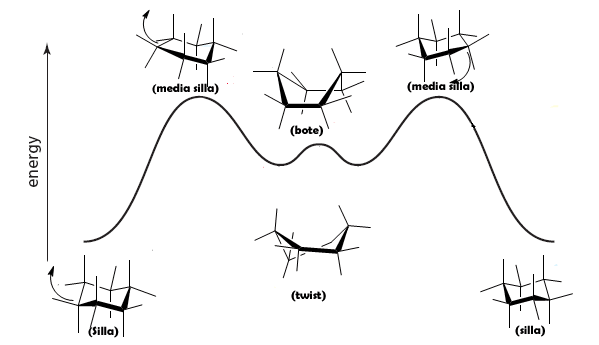
The different conformations of cyclohexane can be represented in an energy diagram: