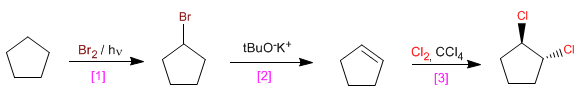Suggest a suitable sequence of reactions to prepare each of the following compounds from the indicated starting material. You can use any organic or inorganic reagent that you consider necessary.
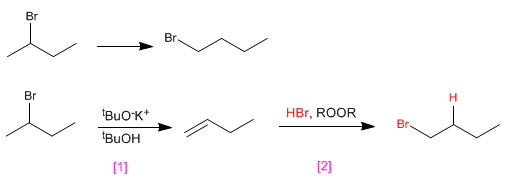
a) 1-Bromobutane from 2-bromobutane
b) 1,2-Dibromopropane from 2-bromopropane
c) 1-Bromo-2-propanol from 2-propanol
d) 1,2-Epoxypropane from 2-propanol
e) Cyclopentyl iodide from cyclopentane
f) trans-1,2-Dichlorocyclopentane from cyclopentane.
SOLUTION:
a) 1-Bromobutane from 2-bromobutane
b) 1,2-Dibromopropane from 2-bromopropane
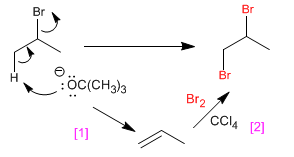
[1] Elimination with strong base to form the alkene.
[2] Anti halogenation of alkene with Br2 /CCl4
c) 1-Bromo-2-propanol from 2-propanol
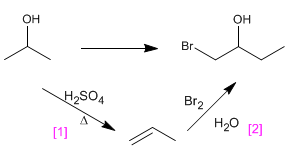
[1] Dehydration of alcohol, to form the alkene.
[2] Formation of bromohydrin by reaction of alkene with Br2 /H2O
d) 1,2-Epoxypropane from 2-propanol
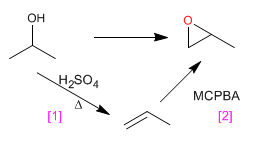
[1] Dehydration of alcohol, to form the alkene.
[2] Alkene epoxidation with MCPBA
e) Cyclopentyl iodide from cyclopentane
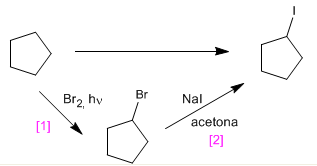
[1] Radical halogenation of the alkane. It cannot be done with I2 /light.
[2] Bimolecular nucleophilic substitution. Elimination followed by addition of HI to the alkene can also be performed.
f) trans-1,2-Dichlorocyclopentane from cyclopentane.