Name the following bicyclic compounds: 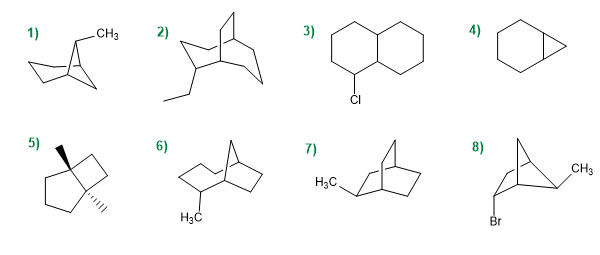
SOLUTION:
Molecule 1. 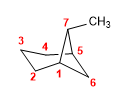
1. Main chain: 7-carbon bicycle (heptane)
2. Numbering: we start from a bridgehead (indifferent) and number the 3-carbon chain first.
3. Substituents: methyl in position 7 (bridge)
4. Name: 7-Methylbicyclo[3.1.1]heptane
Molecule 2. 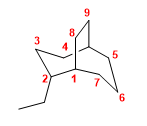
1. Main chain: 9-carbon bicycle (nonane)
2. Numbering: we start from the bridging carbon numbered as 1, so that the ethyl takes the lowest locant.
3. Substituents: ethyl in position 2 .
4. Name: 2-Ethylbicyclo[3.3.2]nonane
Molecule 3.
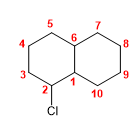
1. Main chain: 10-carbon bicycle (decane)
2. Numbering: we start from the bridging carbon numbered as 1 , so that chlorine takes the lowest locant.
3. Substituents: chlorine in position 2 .
4. Name: 2-Chlorobicyclo[4.4.0]decane
molecule 4
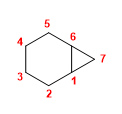
1. Main chain: 7-carbon bicycle (heptane)
2. Numbering: we start with a bridging carbon (indistinct) and number the 4-carbon chain first.
3. Substituents: none.
4. Name: Bicyclo[4.1.0]heptane
Molecule 5. 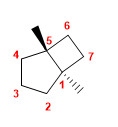
1. Main chain: 7-carbon bicycle (heptane)
2. Numbering: we start from a bridgehead (indifferent) and number the 3-carbon chain first.
3. Substituents: methyls in position 1,5
4. Name: trans-1,5-Dimethylbicyclo[3.2.0]heptane
Molecule 6.
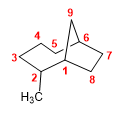
1. Main chain: 9-carbon bicycle (nonane)
2. Numbering: we start with the bridgehead carbon that gives methyl the lowest locant ( 2 ).
3. Substituents: methyl in position 2 .
4. Name: 2-Methylbicyclo[4.2.1]nonane.
Molecule 7. 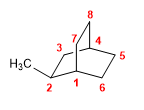
1. Main chain: 8-carbon bicycle (octane)
2. Numbering: Numbering starts from the bridgehead carbon which gives methyl the lowest possible localizer.
3. Substituents: methyl in position 2 .
4. Name: 2-Methylbicyclo[2.2.2]octane
Molecule 8. 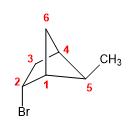
1. Main chain: 6-carbon bicycle (hexane)
2. Numbering: we start from the bridgehead that, when numbering the large, medium and small chain, gives the substituents the smallest locants.
3. Substituents: bromo in position 2 and methyl in position 5 .
4. Name: 2-Bromo-5-methylbicyclo[2.1.1]hexane.