The maximum number of stereoisomers that a molecule presents can be calculated with the formula (2n), where n represents the number of asymmetric carbons. Thus, a molecule with 2 chiral centers has 4 stereoisomers.
Example 1. Draw the possible stereoisomers of 2-Bromo-3-chlorobutane.
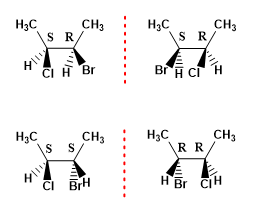
2-Bromo-3-chlorobutane presents 4 stereoisomers by having two chiral centers. These four stereoisomers are classified into two pairs of enantiomers.
Example 2 . Draw the stereoisomers of 1,2-Dimethylcyclohexane.
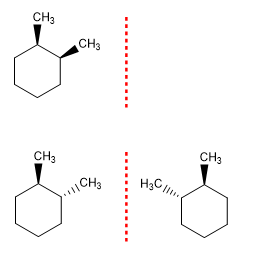
1,2-Dimethylcyclohexane has two chiral centers. Therefore, the maximum number of stereoisomers is: 2n = 4. The symmetry of the molecule can mean that some stereoisomers do not exist. Thus, cis-1,2-dimethylcyclohexane lacks an enantiomer as it is a meso form and the number of stereoisomers is 3.