Benzene reacts with halogens in the presence of Lewis acids to form halogenated derivatives.
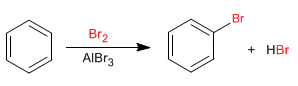
The halogenation mechanism takes place with the following stages:
Stage 1. The bromine molecule becomes polarized by interacting with the Lewis acid. The benzene attacks the positively polarized bromine to form the cyclohexadienyl cation.
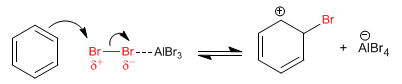
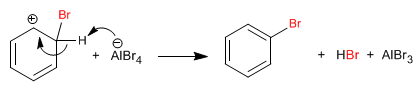
Stage 2. Recovery of aromaticity by loss of a proton.
Chlorination can be carried out in a similar way to bromination. The reaction with fluorine and iodine is carried out very infrequently. In the case of fluorine, the reaction is difficult to control due to its high reactivity. In contrast, iodine reacts slowly and has an unfavorable balance.