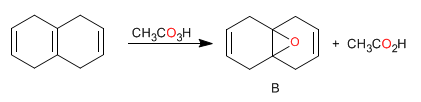
A single epoxide was isolated in 79-84% yield in the following reaction. Is this epoxy A or B? Reason the answer.
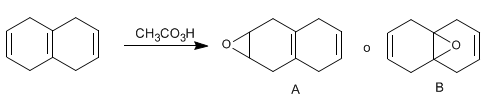
SOLUTION:
Peracids react with alkenes to form epoxides (oxacyclopropanes). When the molecule presents two or more double bonds, the peracid presents a certain chemoselectivity, reacting mainly with the more substituted alkene. It is necessary to use an equivalent of reagent.