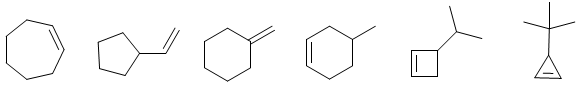
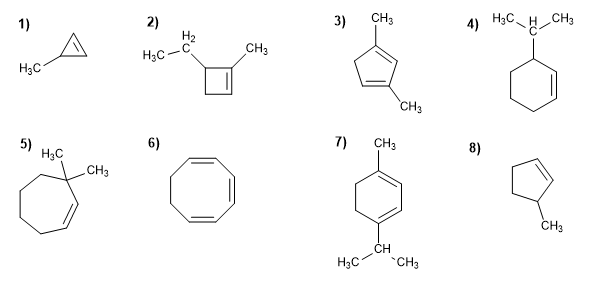
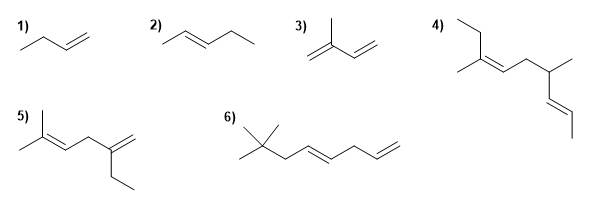
ALKENE PROBLEMS
- Details
- Germán Fernández
- ALKENE PROBLEMS
- Hits: 17663
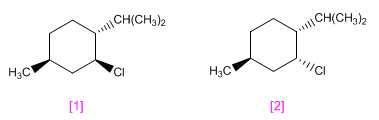
menthyl chloride and that of neomenthyl They have the structures shown below.
- Details
- Germán Fernández
- ALKENE PROBLEMS
- Hits: 54883
Draw the formulas of the following compounds:
| 1) 2-Methylhexa-1,5-diene 2) 2,3,4-Trimethylocta-1,4,6-triene 3) 4-tert-Butyl-2-chlorohept-1-ene 4) 3-Ethyl-2,4-dimethylhept-3-ene 5) 3,4-Diisopropyl-2,5-dimethylhex-3-ene 6) Hept-1-ene 7) cis-Oct-3-ene 8) 3-ethylpent-2-ene |
9) trans-1,4-Dibromobut-2-ene 10) 3-Chlorohex-2-ene 11) Buta-1,3-diene 12) Hexa-1,4-diene 13) 5-Methyl-3-propylocta-1,4,6-triene 14) 6-Methyl-6-propylnone-2,4,7-triene 15) 2,3,5-Trimethylocta-1,4-diene 16) 3-Propylhepta-1,5-diene. |
- Details
- Germán Fernández
- ALKENE PROBLEMS
- Hits: 12010
In the elimination reaction of 5-bromononane with potassium ethoxide in ethanol, draw Newmann projections showing the conformation leading to cis-4-nonene and trans-4-nonene, respectively. Indicate the hydrogen that is lost in each case and suggest a mechanism that explains the observed stereoselectivity.
- Details
- Germán Fernández
- ALKENE PROBLEMS
- Hits: 9292
Write the mechanism that explains the following transformation:
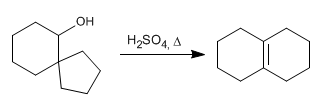
- Details
- Germán Fernández
- ALKENE PROBLEMS
- Hits: 10835
Rank the following alkenes in order of decreasing stability: a) 1-pentene; b) (E)-2-pentene; c) (Z)-2-pentene; d) 2-methyl-2-butene.
- Details
- Germán Fernández
- ALKENE PROBLEMS
- Hits: 9070
Despite attempts, the alkene 2,2,5,5-tetramethyl-3,4-(dimethylethyl)-3-hexene has never been synthesized. Because?.
- Details
- Germán Fernández
- ALKENE PROBLEMS
- Hits: 11795
Write the structure of the three alkenes that are obtained in the acid-catalyzed dehydration of 2-pentanol.
- Details
- Germán Fernández
- ALKENE PROBLEMS
- Hits: 10106
Draw the compound of molecular formula C7H13 Br that each alkene shown as unique elimination product E2 forms.