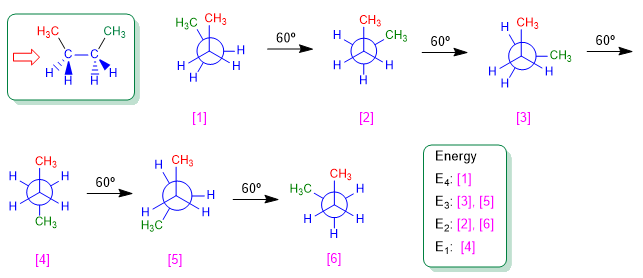
Let's consider the C2-C3 bond of butane. The 60º turns around this link will generate the possible conformations of butane. There are three conformations of special importance called; butane syn , butane anti and butane gauche that we represent in the following models.
The "anti" conformation of butane is the one with the lowest energy. The methyls are on opposite sides, minimizing repulsions. 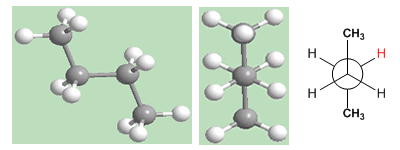
In the gauche conformation, the proximity of the methyls gives rise to a repulsive interaction, called the gauche interaction. This repulsion between methyls is about 3.2 Kcal/mol. 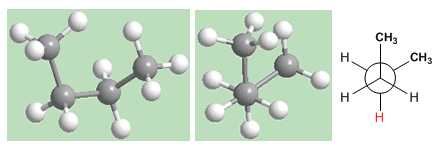
The "syn" conformation is the one with the highest energy. The methyls face each other and the repulsions (eclipse interactions) make this conformation have an energy 25 KJ/mol higher than the anti conformation. 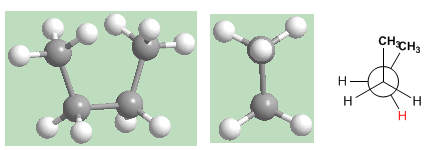
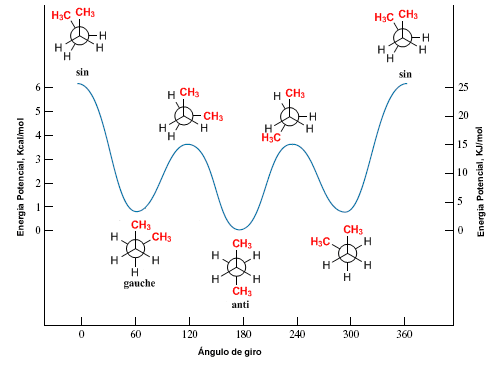
We draw the butane conformations that result from 60º turns.
The diagram of potential energy against the angle rotated for butane leaves us: