Molecular vibrations can be studied with the quantum harmonic oscillator model. The energy is given by:
\begin{equation}\label{energy-oscillator} E_v=\left(v+\frac{1}{2}\right)h\nu \end{equation}
The different energy levels are given by the quantum number v, which takes values 0.1.2.3.4.....
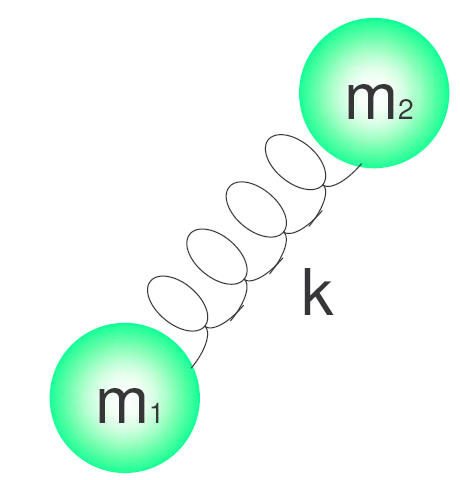
h is Planck's constant and $\nu$ is the frequency of the oscillator which is given by the expression:\begin{equation} \nu=\frac{1}{2\pi}\sqrt{\frac{k}{\ mu}} \end{equation} Where k is the force constant of the spring and $\mu$ is the reduced mass of the system. $\mu=\frac{m_1m_2}{m_1+m_2}$.
Dividing the frequency by the speed of light gives number of waves $\bar{\nu}$ \begin{equation}\label{number-of-waves} \bar{\nu}=\frac{1}{2\pi c}\sqrt{\frac{k}{\mu}} \end{equation} The study of the equation (\ref{number-waves}) will allow us to predict at what number of waves the bonds of a molecule absorb infrared radiation . This equation is only applicable to tension vibrations.
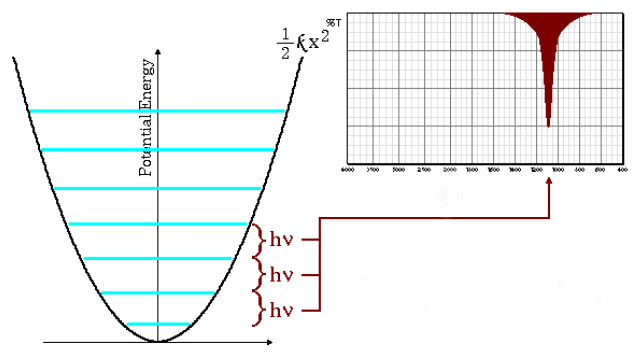
High absorption frequencies
The equation (\ref{wave-number}) indicates that small reduced masses (low mass atoms) and high force constants (strong bonds) lead to high frequencies. Under these conditions the absorption bands emerge at high wavenumbers.
As can be seen in the graph, high frequencies give rise to a greater spacing between energy levels.
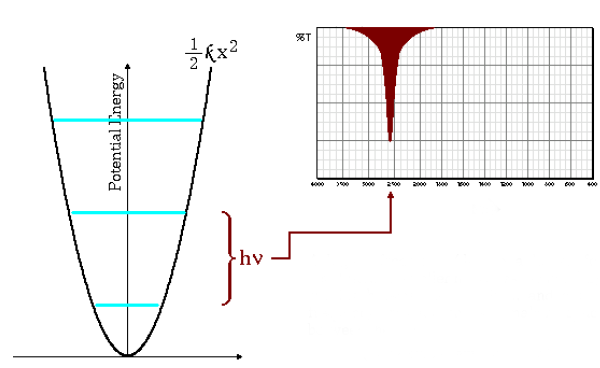
Low absorption frequencies
The equation (\ref{wave-number}) indicates that large small masses and small force constants (weak bonds) lead to low frequencies. Under these conditions the absorption bands come out at low wave numbers.
As can be seen in the graph, low frequencies give rise to less spacing between energy levels.