SYNTHESIS OF HETEROCYCLES (I)
- Details
- Germán Fernández
- SYNTHESIS OF HETEROCYCLES (I)
- Hits: 1203

In this topic we will study reactions in which heterocyles are generated by cyclization reactions. The formation of a cycle can take place from a molecule that contains a nucleophilic center and another electrophilic one, which unite forming a bond. But cycles can also be obtained from two molecules, in this case two nucleophilic centers are necessary in one of them and two electrophilic centers in the other, or each one contains an electrolyle and nucleophilic center. When we speak of a nucleophilic center, we usually refer to an atom with lone pairs and capable of attacking a center of positive polarity (electrophile) that can be found in the same molecule or in another.
- Details
- Germán Fernández
- SYNTHESIS OF HETEROCYCLES (I)
- Hits: 1527
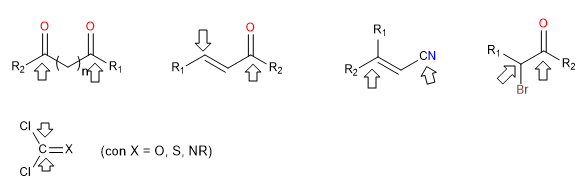
i) Doubly electrophilic reagents
They are reagents that have two positions with strong positive polarity to which nucleophiles attack. These reagents form cycles when confronted with others that are doubly nucleophilic. These are dicarbonyls, alpha, beta-unsaturated, alpha-halogenated carbonyls.
- Details
- Germán Fernández
- SYNTHESIS OF HETEROCYCLES (I)
- Hits: 1603
Nucleophilic attacks are classified based on the hybridization of the electrophilic center (tet (sp3), trig (sp2) and dig (sp). And in exo and endo ring closures, depending on whether the bond broken during the closure of the ring is inside (endo) or outside (exo) of the ring that is formed.
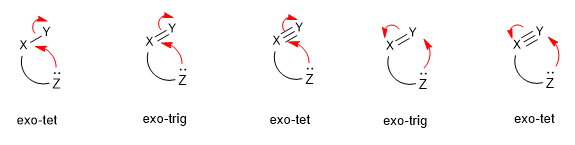
Read more: Classification of nucleophilic attacks (Baldwin's Rules)
- Details
- Germán Fernández
- SYNTHESIS OF HETEROCYCLES (I)
- Hits: 1801
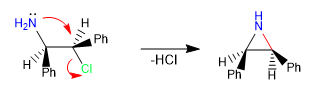
Intramolecular SN2 -type reactions are a powerful tool to form 5, 6, and 3-membered cycles, resulting slow in 4- and 7-membered cyclizations. These are concerted and stereospecific reactions, which further increases their synthetic value.
i) Formation of aziridine.
- Details
- Germán Fernández
- SYNTHESIS OF HETEROCYCLES (I)
- Hits: 2204
In this section we will see examples of synthesis of heterocycles by nucleophilic attack on carbonyl groups. Aldehyde and ketone carbonyls give rise to nucleophilic addition reactions, while carbonyls of acid derivatives (halides, anhydrides, esters, amides, nitriles) give rise to addition-elimination processes.
A) We start with the synthesis of a benzofuran.
In the first stage we join the reactants by means of an SN2 and we end up cycling with an aldol reaction.
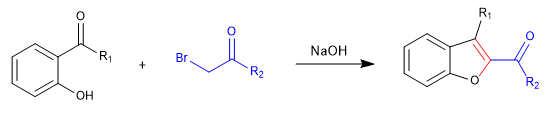
Mechanism:
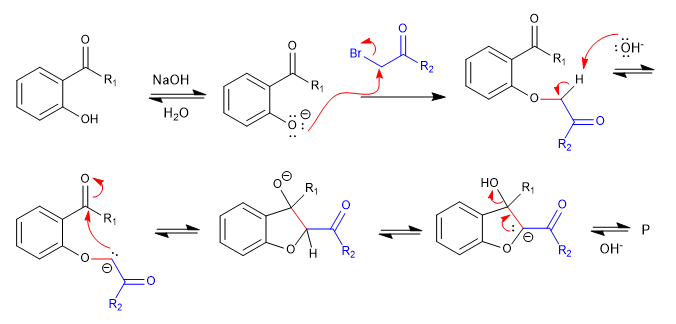
Read more: Cyclization processes by nucleophilic addition and addition-elimination at $sp^2$ carbons
- Details
- Germán Fernández
- SYNTHESIS OF HETEROCYCLES (I)
- Hits: 1708
Alkynes and nitriles participate as electrophiles in numerous heterocycle synthesis reactions, being attacked by a wide variety of nucleophiles.
A) The nucleophilic-electrophilic character of propanedinitrile and alpha-aminoketones allows the preparation of pyrroles , let's see an example.
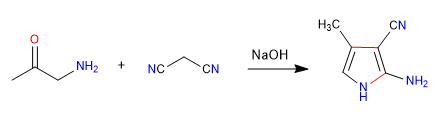
Mechanism:
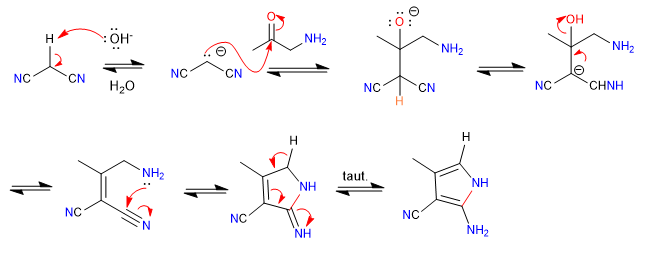
- Details
- Germán Fernández
- SYNTHESIS OF HETEROCYCLES (I)
- Hits: 2303
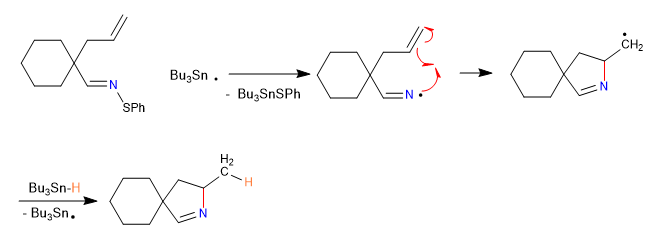
In this section we will see examples of cyclizations that take place through intermediates of radical type, carbene or nitrene.
A) We begin with a radical cyclization, which uses tributyltin hydride as a reagent and is initiated by AIBN
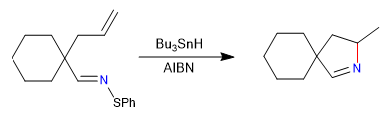
Mechanism:
1. initiation
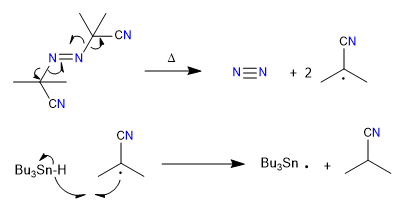
2. Propagation