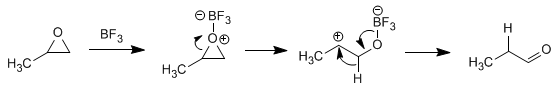HETEROCYCLES OF 3, 4 AND 7 MEMBERS
- Details
- Germán Fernández
- HETEROCYCLES OF 3, 4 AND 7 MEMBERS
- Hits: 8829
The properties of three-membered rings are related to strain, due to the low bond angles. Angular strain gives rise to opening reactions to form acyclic products.
In this section we will study the following heterocycles: oxirane, thiirane, 2H-azirine, aziridine, dioxirane, oxaziridine, 3H-diazirine and diaziridine.
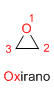
Boiling point: 10.5ºC
Ring stress: 114 KJ/mol
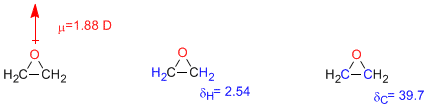
dipole moment: 1.88 D
Characteristics: Colorless liquid, soluble in water, very poisonous in the gas phase.
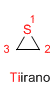
Boiling point: 55ºC
Ring stress: 83 KJ/mol
dipole moment: 1.66 D
Characteristics: Colorless liquid, partially soluble in water.
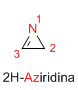
Boiling point:
Ring stress: 170 KJ/mol.
dipole moment:
Characteristics: Very unstable substance, with high reactivity in the C=N bond
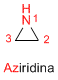
Boiling point: 57ºC
Ring stress: 113 KJ/mol.
dipole moment: 1.89 D
Characteristics: Colorless liquid, soluble in water, poisonous, with an ammonia-like odor.
- Details
- Germán Fernández
- HETEROCYCLES OF 3, 4 AND 7 MEMBERS
- Hits: 13525
Spectroscopic data: UV spectrum: λ=171 nm. NMR : δH= 2.54, δC= 39.7
- Details
- Germán Fernández
- HETEROCYCLES OF 3, 4 AND 7 MEMBERS
- Hits: 31309
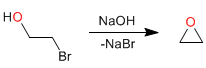
Mechanism:
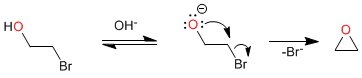
- Details
- Germán Fernández
- HETEROCYCLES OF 3, 4 AND 7 MEMBERS
- Hits: 29656
Oxiranes act as Lewis bases, protonating on oxygen in acidic media. The second type of reactivity is related to ring strain that favors opening reactions.
Isomerization to carbonyl compounds: Oxiranes in the presence of Lewis acids isomerize to form aldehydes or ketones. Thus, oxirane in the presence of BF 3 is transformed into acetaldehyde
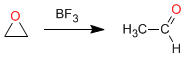
Example: Propose a mechanism for the following transformation:
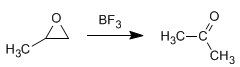
Solution - The BF 3 protonates the oxygen of the oxirane, turning it into a good leaving group and favoring the opening of the cycle. A rearrangement to a hydroxycarbocation will give the expected product.