INDOL THEORY
- Details
- Germán Fernández
- INDOL THEORY
- Hits: 4402
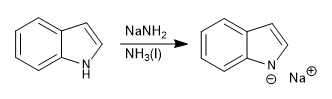
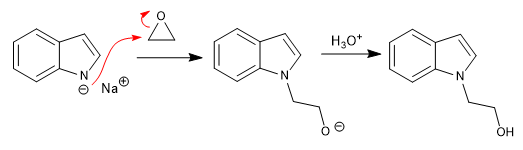
The indole presents an acidic hydrogen on the nitrogen atom, with pKa= 17. Using strong bases it can be deprotonated, forming the indolium anion.
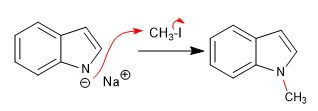
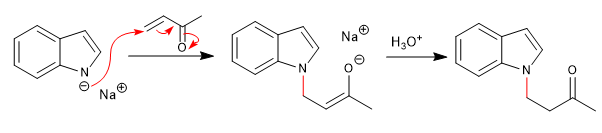
Reaction of indolium with different electrophiles:
a) Rental:
b) Opening of epoxides:
c) Attack to a,b -unsaturated:
- Details
- Germán Fernández
- INDOL THEORY
- Hits: 7317
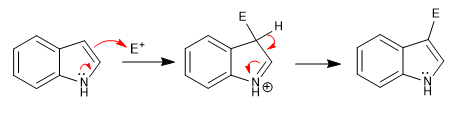
The attack on the electrophiles occurs from position 3, because the pyrrole ring is richer in charge density than the carbocycle. Said attack is favored by the transfer of the lone pair of nitrogen.
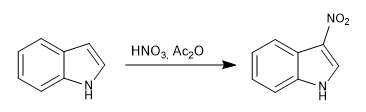
a) Nitration:
The indole is nitrated with the nitric mixture in acetic anhydride.
- Details
- Germán Fernández
- INDOL THEORY
- Hits: 2135
The 3-substituted indoles add the electrophile to position 3, rearranging it to position 2 at a later stage.
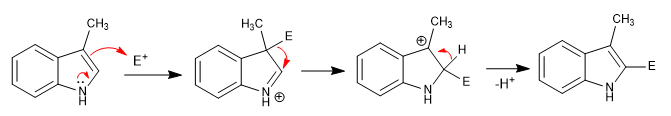
Read more: Electrophilic substitution with position 3 of the indole occupied
- Details
- Germán Fernández
- INDOL THEORY
- Hits: 2137
a) Leaving groups near the ring
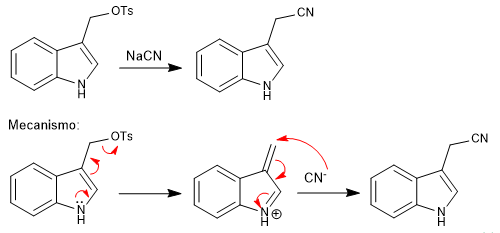
- Details
- Germán Fernández
- INDOL THEORY
- Hits: 3246
The indole is converted to quinoline by reaction with a carbene generated by a 1,1 elimination from chloroform. The carbene undergoes a cyclopropanation reaction on the double bond of the pyrrole ring, opening the cyclopropane generated at a later stage, to generate the final quinoline.
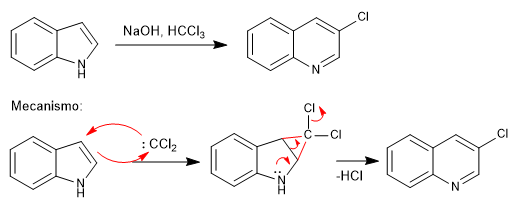
- Details
- Germán Fernández
- INDOL THEORY
- Hits: 3410
We can selectively reduce either the carbocycle or the pyrrole ring.
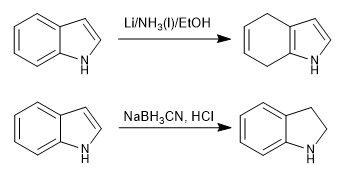
- Details
- Germán Fernández
- INDOL THEORY
- Hits: 3187
It is one of the most important synthesis of indole. Part of phenylhydrazine and a carbonyl with alpha hydrogens, which generate a hydrazone. After a tautomerism, a sigmatrope is produced that yields the indole after cyclization.
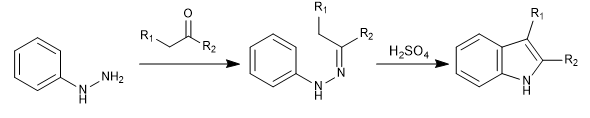
The hydrazone formation step has a well-known mechanism (the same one that forms imines) and we are not going to comment on it. We will focus on the steps that convert hydrazone to indole.
Stage 1. Tautomerism.
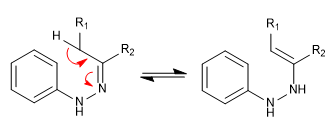
- Details
- Germán Fernández
- INDOL THEORY
- Hits: 3700
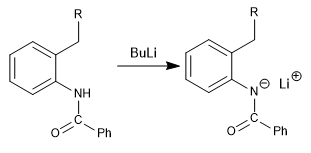
The Madelung synthesis is based on the formation of a benzylic carbanion that cyclizes on an amide substituent.
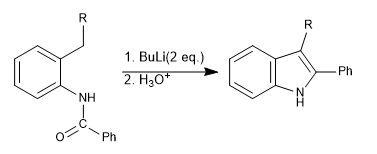
Mechanism:
Step 1. Deprotonation of the amide