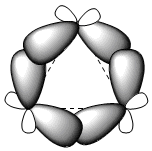
 The three carbon atoms of cyclopropane lie in the same plane, unlike the other cycloalkanes which are not planar. Cyclopropane is characterized by a large angular strain due to bond angles well below 109.5°. The enormous strain to which the cycle is subjected causes the carbon-carbon bonds to bend outwards, giving rise to very characteristic bonds called "banana" bonds.
The three carbon atoms of cyclopropane lie in the same plane, unlike the other cycloalkanes which are not planar. Cyclopropane is characterized by a large angular strain due to bond angles well below 109.5°. The enormous strain to which the cycle is subjected causes the carbon-carbon bonds to bend outwards, giving rise to very characteristic bonds called "banana" bonds.
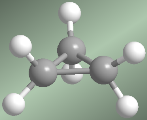 Cyclopropane has three hydrogens facing each other (eclipsed hydrogens), which further increase the tension of the molecule. It is for this reason the most unstable cycloalkane.
Cyclopropane has three hydrogens facing each other (eclipsed hydrogens), which further increase the tension of the molecule. It is for this reason the most unstable cycloalkane.









