SN2 reactions with leaving groups in allylic positions proceed more rapidly than those for the corresponding saturated haloalkanes.
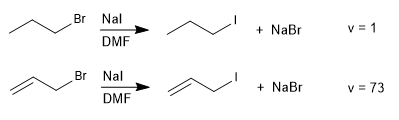
Allylic systems with good nucleophiles and in aprotic solvents give SN2 reactions. The mechanism is identical to that studied in haloalkanes, only a higher reaction rate is observed due to the stabilization of the transition state by the double bond.