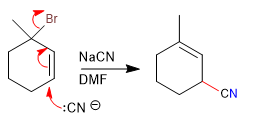
With tertiary allylic substrates and under SN2 conditions (good nucleophile and aprotic solvent), a concerted reaction is produced by the nucleophile attacking the carbon of the double bond with loss of the leaving group.
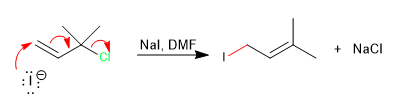
The attack occurs on the double bond since it is a less hindered position than the chlorine carbon.