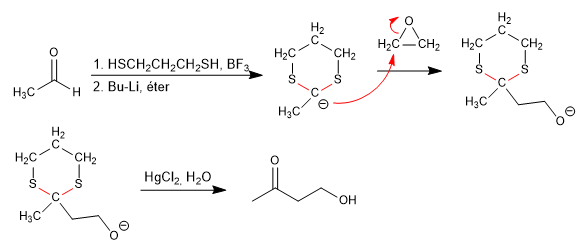
The reaction of aldehydes with 1,3-dithiacyclohexane, in the presence of Lewis acids, produces 1,3-dithianes that have hydrogens that can be subtracted with strong bases, forming the 1,3-dithiane anion. These anions are very good nucleophiles and react with a wide range of electrophiles.
Let's see how to obtain 2-butanone from ethanal.
Mechanism:
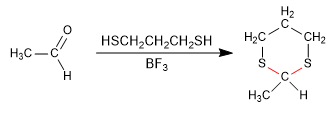
Stage 1. Formation of 1,3-dithiane
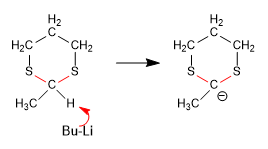
Stage 2. Formation of the 1,3-dithiane anion.
Stage 3. Nucleophilic attack
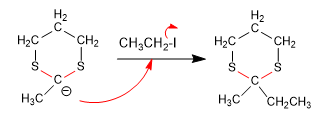
Stage 4. Hydrolysis of the thioacetal
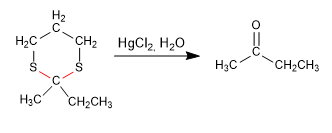
Other examples: