Positive, negative or radical charges in positions close to double bonds are delocalized by resonance, being especially stable.
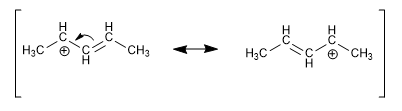
Allylic carbocation
The carbocation formed in neighboring positions of double bonds is known as an allylic carbocation. It is stabilized by delocalizing the charge between two carbons, and is therefore more stable than a normal secondary carbocation.
Conjugated dienes are molecules that have several double bonds alternating with single bonds. This type of structure allows the movement of p electrons throughout the entire molecule, allowing their delocalization and increasing the stability of the compound.
Conjugated dienos:
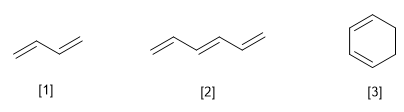
[1] Buta-1,3-diene
[2] Hexa-1,3,5-triene
[3] Cyclohexa-1,3-diene
Unconjugated dienos:
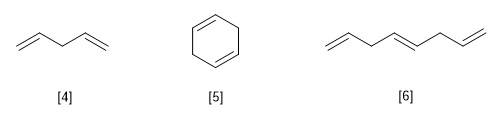
[4] Penta-1,4-diene
[5] Cyclohexa-1,4-diene
[6] Octa-1,4,7-triene
Conformational equilibrium in dienes
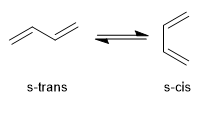
1,3-Butadiene can adopt two possible conformations called s-cis and s-trans that interconvert by rotation. This balance is important in the Diels-Alder reaction since only the s-cis conformation is reactive.

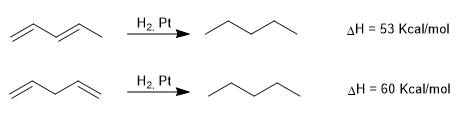
Heats of hydrogenation in conjugated and non-conjugated dienes

As can be seen, the conjugated diene releases less heat when hydrogenated, because it contains less energy, that is, it has greater stability.