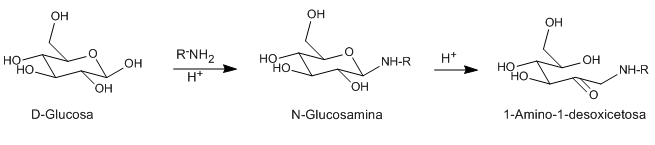
Acids and bases catalyze the isomerization of N-glycosides (glycosamines) from aldoses to 1-aminodeoxyketoses. This rearrangement can be catalyzed with a number of Lewis acids (CuCl 2 , MgCl 2 , AlCl 3 , SnCl 4 ). The amine used as a reagent can be primary or secondary, aliphatic or aromatic.
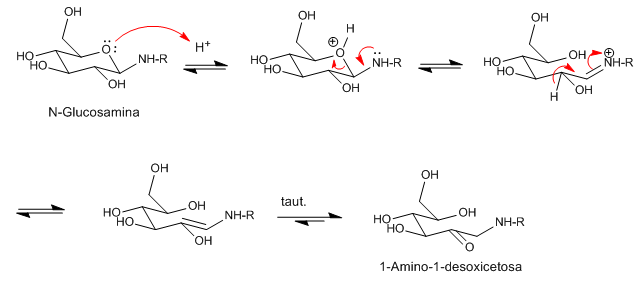
The mechanism consists in the coordination of the Lewis acid on the ring oxygen atom producing the opening of the ring. The loss of a proton generates an enolic-type intermediate that yields 1-Amino-1-deoxyketose by tautomerism.