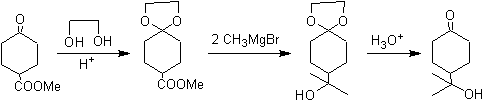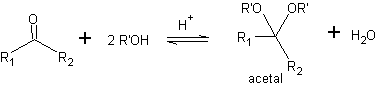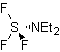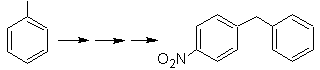ORGANIC SYNTHESIS
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 45885
ORGANIC SYNTHESIS METHODOLOGIES
The total synthesis of an organic compound would require starting each time from the elements that compose it. However, it is well known that simple organic compounds such as urea, methane, methanol, acetylene, acetic acid, ethanol can be obtained from the elements, and so on, increasingly complex structures can be built.
However, this is neither practical nor necessary as there are a large number of organic compounds that are commercially available or economically available and these can be used as starting materials. Strictly speaking, all of them derive from the elements that make them up or can be derived from them, so any synthesis that is undertaken from these raw materials will be “formally” a total synthesis.
The synthesis methodologies to face a successful synthesis have been changing with the passage of time and the development of chemistry itself as a science, hence the following are known:
- Methodology of the “direct association”
- Methodology of the “intermediate approach”
- Methodology of "logical analysis"
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 30911
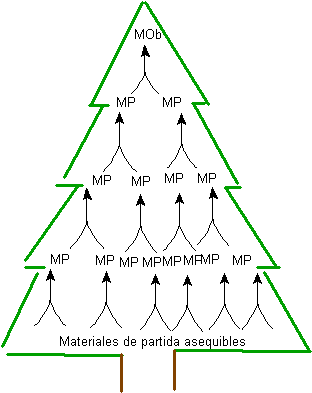
The elaboration of a " synthesis tree " based on generating intermediate or precursor molecules, step by step in the antithetic direction (retrosynthesis), that is, starting from the objective molecule, constitutes a method that can be better understood by considering the following general principles of said process.
1. Start with the final structure (MOb). Starting from the final structure, the target molecule, work backwards (retrosynthesis) until easily accessible raw materials are obtained. If the starting raw material is specified in the synthesis problem, this only limits the number of possible synthetic routes to be addressed.
2. Characterization of the target molecule (MOb ). When examining the structure of the target molecule, it is necessary to answer the following questions:
to. What kind of compound is it?
b. What functional group(s) does it contain?
c. What is the nature of the carbon skeleton?
d. Does the molecule have a normal or branched alkyl chain?
and. Does it contain rings and are they cycloalkyl or aromatic?
F. Does the MOb have actual or potential symmetry?
3. The Functional Group . In this regard, it will also be good to answer the following questions:
to. Is the reactivity, sensitivity and instability of the functional groups that the MOb possesses known?
b. What general methods are available for its preparation?
c. Which of them is applicable to the specific functional group of the problem molecule?
4. Stereochemical aspects . It will be analyzed in the MOb, preferably:
to. chirality centers
b. Shaping and configuration of rings
c. Proximity effects between groups
5. The carbonate skeleton . The main problem in most organic syntheses is the construction of the carbon skeleton. The exchange of functional groups (IGFs) is often simple to do, such as ketone to alcohol, aldehyde to acid, or alcohol to bromide. The questions that are asked regarding the construction of CC links are related to those that have already been raised regarding the functional group.
to. Are some of the methods available to form functional groups applicable to generate CC links? If so.
b. Is the method compatible with the specific carbon skeleton of the target molecule? If it is not.
c. Is there a procedure for forming a carbon chain that produces a convertible function to the required one?
6. Precursor Molecules (PM)
The analysis of the structure of the problem molecule and the consideration of the questions posed in steps 1) to 5), will give rise to two possible types of precursor molecules. One of them contains a functional group equivalent to that of the final structure.
The other is a compound with fewer carbon atoms than the target molecule. When the latter are brought together, the final carbon chain and the required functionality are achieved.
The generation of any of these types of precursor molecule should result in a simplification of the problem.
In general, if a projected path leads to precursors that are more difficult to synthesize than the problem itself (target), another path must be sought.
Fig. 1. TREE OF SYNTHESIS
The generation of precursor molecules, up to reaching the starting materials, generates a series of structures, which together form a kind of tree, fig.1. This is where the name of the synthetic method comes from.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 36769
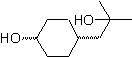
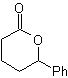
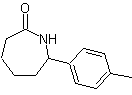
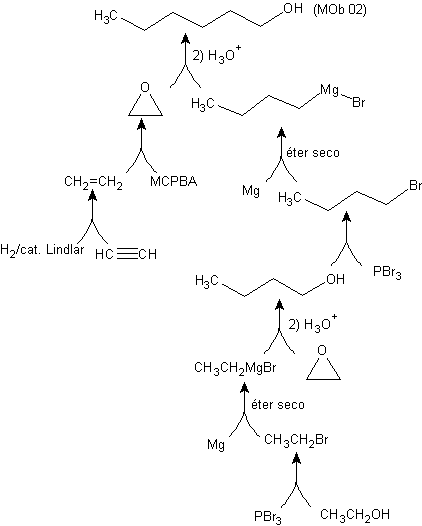
Synthesis of n-Hexanol ( MOb 02)
Solution : n-Hexanol (MOb 02) is a primary alcohol, whose carbon chain has no branches. Therefore, the strategy is reduced to looking for reactions that allow the chain to grow in a good number of carbon atoms. It is not advisable that the growth of the chain is one by one, since this path would lead to a synthesis plan with many stages, consequently a low yield.
As such, the opening of epoxide rings by a Grignard compound can be adequate for this purpose; as it can also be combined with acetylenic synthesis (use of derivatives of sodium acetylide and subsequent saturation of the triple bond).
The epoxide needed to combine with the Grignard is prepared from an alkene and a peracid acid. Thus, the present synthesis plan is deduced, where the starting materials can be acetylene and the ethanol.
Read more: Synthesis problems, solved by the Synthesis Tree method
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 42088
Formation of enols and enolates
The alpha carbon of compounds containing the carbonyl group (aldehydes, ketones, esters, diketones, diesters, nitrates, nitriles, etc.), is the center of many CC bond formation reactions. Due to the acidity of the H a , they undergo a -deprotonation in the presence of a suitable base, with the consequent formation of a carbanion. The resulting negative charge on C a to C=O is resonance stabilized by the same carbonyl group.
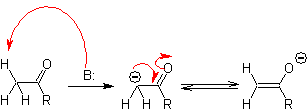
The selection of the base, for the formation of enolates, is subject to the fact that the pKa of the conjugate acid of the base must be greater by at least three units than the pKa of the carbonyl compound that has acidic H 's .
| pK a = 20 | MeO- pK a = 15 | Unfavorable enolate formation |
| pK a = 10 | tBuO- _ pK a = 19 | Very favorable enolate formation |
Formation of enolates:
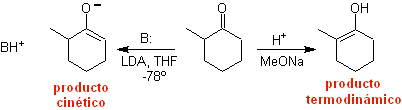
¨ The kinetic enolate
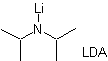
It occurs because the substrate has H α , easily accessible for deprotonation through a typical base such as LDA (pKa
approx 30) LDA (lithium diisopropylamide) is a strong, non-nucleophilic, sterically hindered base. |
|
¨
Enolates of esters:
Esters are susceptible to a substitution reaction for the base, LDA can be problematic, which is why the non-nucleophilic base (lithium isopropylcyclohexyl amide) is used with esters.
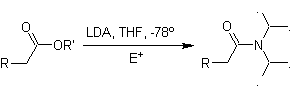
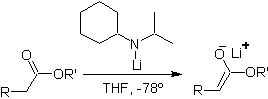
¨
Thermodynamic enolate:
A reversible deprotonation can lead to more stable enolates, which occurs when the more substituted C=C of the enol form is obtained.
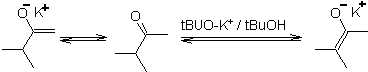
Typical conditions to form thermodynamic enolates are: RO-M+ in ROH as protic solvent (pKa of ROH = 15 to 18).
Kinetic and thermodynamic enolates can be trapped, isolated, separated, and purified to obtain regiochemically pure enolates. This can be accomplished by the formation of enol and silylene ether acetates.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 46146
Reactions of Enols and Enolates
Aldol reactions and the so-called condensation reactions of carbonyl compounds and others of this type, which can form enol and enolate structures, participate in a large group of important reactions that allow us to understand the existence of an immense number of molecules resulting from the interaction of enols or enolates with a series of electrophilic groups.
The study of this type of reaction has made it possible to verify and establish the existence of two reaction mechanisms through which they occur, as explained below:
TO)
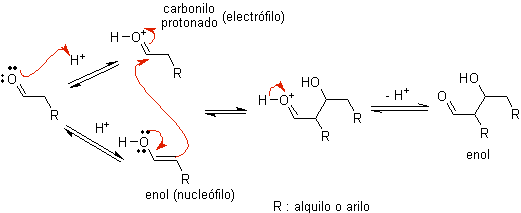
When acid is used as a catalyst, the carbonyl compound is initially protonated and then tautomerized to its enol form , which is a nucleophile on the alpha carbon to the carbonyl group. The same acid medium is enough to activate the carbonyl group of another molecule, making it highly electrophilic, which generates optimal conditions to produce an unsaturated carbonyl compound.
The reaction normally proceeds until the dehydration of the enol formed, catalyzed by the same acid of the reaction.
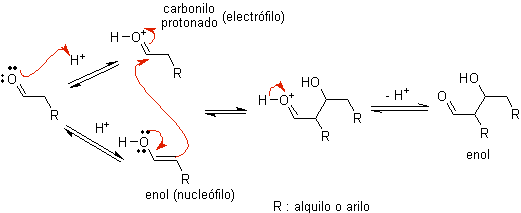
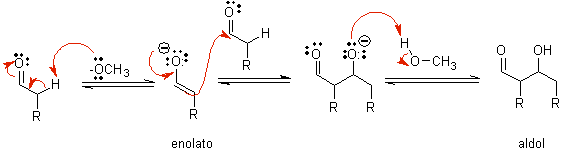
B) When the catalyst is a base, such as an alkoxide, the aldol-type reaction proceeds via the nucleophilic attack of the resonance-stabilized enolate on the carbonyl group of another molecule.
By dehydration of the aldol, catalyzed by base, the dehydrated final product is formed.
As in the previous case, the base-catalyzed dehydration (sometimes written as a single step), allows to control the reaction and produce a dehydrated final product. In some cases, the formation of enolates is irreversible.
how it looks only a catalytic amount of base is required in some cases, the most usual procedure is to use a estequiométrica amount of strong base such as LDA or NaHMDS . In this case, enolate formation is irreversible, and the aldol product is not formed until the metal alkoxide of the aldol product is protonated in a later step.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 36957
Organic synthesis, the heart of organic chemistry , is an essentially heuristic activity, that is, it is a process where the highly predictive creative activities of logical thought and empirical procedures, rich in artistic elaboration, merge, making the organic chemist a true innovative.
Like any method, the " disconnection or synthon method " has its own structure, symbology and language, which must initially be assimilated and understood by those who are willing to use this synthetic tool.
The method of synthesis of the disconnections or synthon , includes Two phases;
![]() Retrosynthetic analysis phase . It shows all the transformations that will be carried out in the process of simplifying the structure of
Retrosynthetic analysis phase . It shows all the transformations that will be carried out in the process of simplifying the structure of
![]() Synthesis phase . Where what is "thought", based on criteria of mechanistic rationality and reactivity of organic compounds, materializes in a synthesis route, which will be written, as it is expected to occur in the chemical laboratory. It is where experience emerges and manifests the " art of doing or invent ” of the chemist, i.e.
Synthesis phase . Where what is "thought", based on criteria of mechanistic rationality and reactivity of organic compounds, materializes in a synthesis route, which will be written, as it is expected to occur in the chemical laboratory. It is where experience emerges and manifests the " art of doing or invent ” of the chemist, i.e.
The terms, definitions or synthesis operations, recurrently used in this method, are the following:
Target Molecule (MOb) .
This is the name given to any molecule that is to be synthesized or prepared from simple and affordable materials, which in a problem may be previously defined or adjusted to the options that the chemist generates in his synthesis plan or design.
transformation . ( ![]() ).
).
The special unidirectional retrosynthesis arrow should be understood as a symbolic representation of the expression “ is prepared from ” and also represents some kind of transformation in the structure of
The types of transformation referred to are actually retrosynthetic operations such as: Disconnections, Reconnections, Rearrangements, Interconversion of Functional Groups (IGF), Addition of Functional Groups (AGF), Deletion of Functional Groups (SGF), etc.
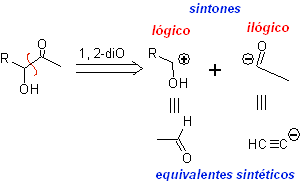
Disconnection.
It is a retrosynthetic operation that represents the imaginary breaking of the chemical bonds that would have been formed in the target molecule, from the synthons or more precisely from their synthetic equivalents (precursor molecules), postulated.
It can be understood as the inverse of a chemical reaction, it is represented by an arrow (very different from that of a chemical reaction or equilibrium conditions) and a crossed wavy line over the bond that will be "disconnected".
It is even possible to place the proposed disconnection on the arrow: CC, CS. CX, CO, CN. etc.. Expressions that link us to the type of reaction that will be used, in the formation of
In other cases, the disconnection model that is being used can be written, for example, it is common to find: 1, 3-diO, 1,4 –diCO, 1,5-diCO or α, β -insatCO . Etc.
Therefore, in a dioxygenated molecule, the following disconnections can be expected to occur:
![]() heterolytic disconnections,
heterolytic disconnections,
![]() Homolytic or radical disconnections
Homolytic or radical disconnections
![]() Electrocyclic Disconnections
Electrocyclic Disconnections
![]() Reorder Disconnects
Reorder Disconnects
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 29647
DISCONNECTIONS OF 1,3-DIOXYGEN COMPOUNDS
Oxygenated organic compounds are the most abundant in nature and particularly dioxygenates, which is why many chemical researchers have modeled the retrosynthetic disconnection of these molecules, an aspect that will be studied in the following paragraphs.
To begin with, the disconnection models of dioxygenated molecules have been divided into two large groups, based on the nature of the synthons that are generated with the application of a basic synthetic operation called " DISCONNECTION" to the molecule to be synthesized and that generally it is referred to as a target molecule (MOb) .
These large groups are:
![]() “ Logical ” disconnection models, and
“ Logical ” disconnection models, and
![]() “ Anomalous ” or “ illogical ” disconnection models
“ Anomalous ” or “ illogical ” disconnection models
The so-called "logical" disconnection models are those that, by applying a "disconnection" of one or several chemical bonds in
Compounds that can be classified as 1,3-dioxygen and 1,5-dioxygen, when subjected to retrosynthesis, generally form synthons considered "logical." On the other hand, organic molecules related to 1,2-dioxygenated, 1.4-dioxygenated and 1,6-dioxygenated, generate synthons
considered “illogical”
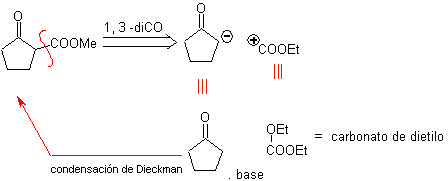
Cut-off model 1,3-dioxygen
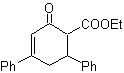
![]() β-dicarbonyl compounds
β-dicarbonyl compounds
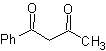
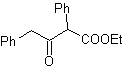
The 1,3-dicarbonyl compounds are obtained with good yields through Claisen-type condensation reactions, which involve the reaction between esters and compounds with active hydrogens, such as: esters, ketones, aldehydes, nitriles, nitroderivatives, and some hydrocarbons in presence of alkaline reagents.
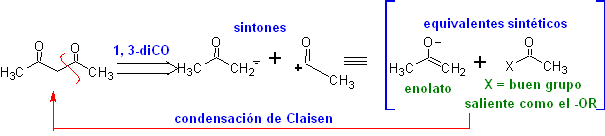
The β-diketones and β-ketoaldehydes are obtained by the crossed Claisen condensation, using a suitable ketone and ester. In the crossed Claisen condensation of ketones and esters, good yields are obtained because ketones are notably more acidic than esters, therefore, in the basic medium, the ketone is deprotonated to a greater degree than the ester.
Examples : Propose a synthesis design from simple materials, for the following molecules:
MOb 16
| mob 17
| MOb 18
|
Solution:
MOb 16 . Apparently the two disconnection alternatives (a) and (b), shown in
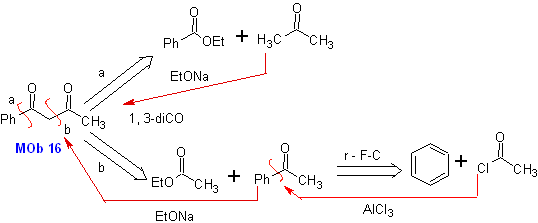
However, alternative (b) turns out to be the most suitable, since, in the basic reaction medium, the carbanion formed PhCOCH 2 - would be better stabilized, due to resonance and inductive effects.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 28102
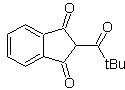
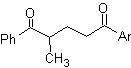
The 1,5-dioxygenated compounds are generally the result of conjugate addition reactions of nucleophiles from carbonyl compounds, with acidic H α (enols, enolates, enamines, etc.), as well as nitriles and nitrates, on substrates alpha beta unsaturated with respect to to carbonyl groups and the like, known as the Michael reaction, with complementary options being the Nef reaction and the Robinson annulation (annulation) reaction.
Disconnect model 1, 5 dioxygen (1,5-diO)
The 1,5-diO disconnection model can be applied, after the necessary functionalization, to compounds such as: 1,5-dihydroxyls, 1,5-hydroxyaldehydes, 1,5-hydroxyketones, 1,5-hydroxyesters, 1, 5-ketoaldehydes, 1,5-diketones, 1,5-ketoesters, 1,5-dialdehydes, etc.
The possibilities increase if nitroderivatives and nitriles are also taken into account, which can form very reactive carbanions in a basic medium capable of adding to α,β-unsaturated carbonyl compounds to obtain 1,5-diO type products.
The fundamental analysis of the disconnection of 1,5-diO compounds is as follows:
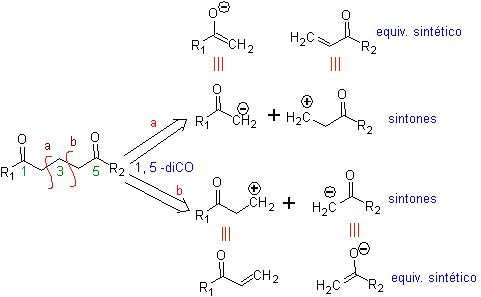
The choice of disconnection (a) or (b), around C3, will depend on the nature of the R1 and R2 groups, which may confer greater or lesser stability to the synthon or synthetic equivalent necessary for the formation of
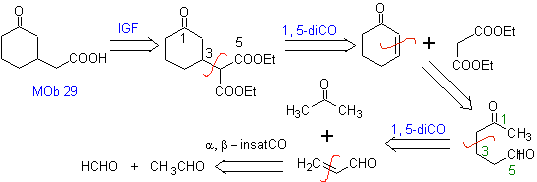
Propose a synthesis design for MOb 29, 30 and
MOb 29
| MOb 30
| MOb 31
|
Solution:
MOb 29 . Retrosynthetic analysis: The carbanion needed to add to the a , b -unsaturated compound CO It can be obtained from diethyl malonate in a basic medium. Which will subsequently force a decarboxylation, to reach
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 61749
The protection operation requires the following procedure:
·
Protect the most reactive functional group(s) selectively and under mild conditions.
·
Carry out the reaction on the required functional group without affecting the protected group
·
Unprotect functional group, subjected to protection
The protection action must satisfy the following basic requirements:
·
The reaction must perform well and be chemoselective.
·
The new functional group must be stable under the reaction conditions of the group that will react.
·
The introduced functionality must not add chiral centers to the molecule that can generate diasteromers
·
The original functional group must be able to be regenerated with good yield and without affecting the rest of the molecule.
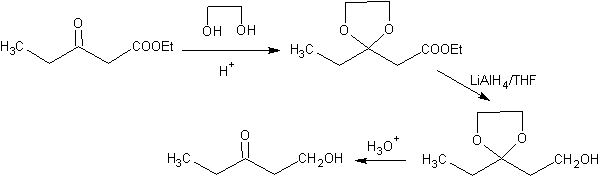
The use of protectors should be reduced to the essential minimum and their choice should be such that they do not need to be replaced throughout the synthesis, since the introduction and removal steps (deprotection) add cost and work to the synthesis and decrease the yield. Example.
The ketonic group of the molecule has been protected by transforming it into a cyclic ketal, with an ethanediol in a slightly acid medium, subsequently this molecule has been reacted with two moles of phenyl magnesium bromide, which acts on the ester group, to transform it into a tertiary alcohol, with two methyl substituents contributed by the Grignard. Finally, the cyclic ketal is hydrolyzed to regenerate the ketone.
In practice, there is no perfect protective group for each functionality, instead it can be affirmed that there is a large battery of possible protectors, each of which meets the above conditions under certain circumstances. A short list of protection of the most common groups is included in the following sections:
PROTECTION FROM ALDEHYDES AND KETONES
One way of protecting ketones and aldehydes is their conversion to acetals. Acetals can be deprotected under mild conditions by acid hydrolysis reactions.
In the reduction of a ketoester to ketoalcohol. Protection of the ketone in the form of an acetal is very convenient because the acetal resists the reducing conditions under which it will be used in the conversion of the ester group to a hydroxyl group.
The following scheme shows the complete synthesis sequence that allows achieving the reduction of the ester without affecting the ketone:
In the first stage, the ketone is converted to a cyclic acetal by reaction with ethylene glycol in the presence of an acid catalyst. In the second stage, the ester is reduced with LiAlH 4 . This reagent does not attack the acetal. Finally, in the third stage, the alcohol-acetal is treated in an aqueous acid medium. Under these conditions, the acetal is hydrolyzed, regenerating the ketonic carbonyl group. Each of the three stages is chemoselective since in each of them the preferred reaction of a functional group is achieved.
in the presence of another.
Read more: Protection of Functional Groups in Organic Synthesis
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 30533
Control in organic synthesis is one of the most important tasks to achieve the required or planned transformation. and/or to avoid the formation of those by-products that substantially impair the optimal development of the organic synthesis in question. Likewise, from a more general perspective, the control can also include or cover the aspects of symmetry and selectivity.
Then, control should be understood as a series of synthetic operations that allow the chemist to form the carbon skeleton with the intended functionality or to "place" a group or atom in the required place or position.
Consequently, these operations may be of a varied range of routines with an intention reflected by the chemist and that demand certain cognitive abilities and skills similar to artistic ones, for the construction (synthesis) of organic molecules.
Therefore, in condensation reactions, as in others, control operations may be included in one of the following categories.
![]() Competing reactions (self-condensation and/or cross-condensation)
Competing reactions (self-condensation and/or cross-condensation)
![]() Activation – deactivation
Activation – deactivation
![]() Selectivity and specificity
Selectivity and specificity
![]() Protection-deprotection
Protection-deprotection
In the condensation reactions of carbonyl compounds, it is essential to establish the order of events in advance to minimize or, if necessary, suppress the possibilities of self-condensation and the occurrence of cross-condensation, which unfortunately are an evident threat in these reactions.
self condensation
All carbonyl compounds that have one or more alpha hydrogens, on the carbons adjacent to the carbonyl group, run the risk of suffering a self-condensation reaction if the corresponding rigor is not followed.
Thus, for example, if a non-hydroxylated base such as EtONa is added to a 2-butanone, an enolate will be formed that could eventually combine with another molecule of the same ketone. Similarly, if H+ is added, self-condensation can also occur, as can be seen in the following scheme: This can be minimized by adding at the same rate, from separate feeders, the base or acid to the compound that will provide the enol or enolate and the carbonyl compound that will be attacked on the carbonyl carbon. A three-necked flask and magnetic stirring can be used.
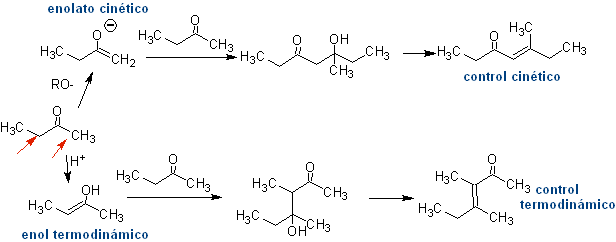
cross condensation
Ideally, in condensation reactions of carbonyl compounds, one of the reacting molecules should enolize rapidly, while the other should preferably have no Hα. , to ensure that no other by-products are formed
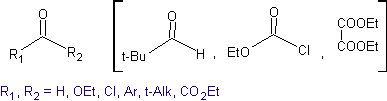
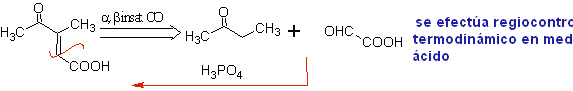
The retrosynthesis that is analyzed below is a good example of what was previously indicated.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 28093
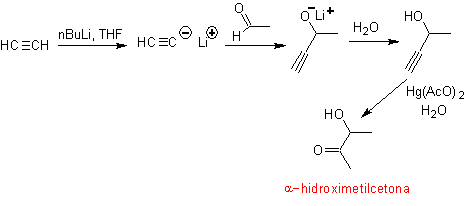
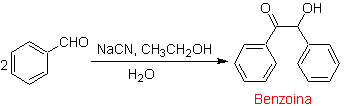
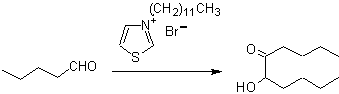
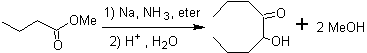
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 29810
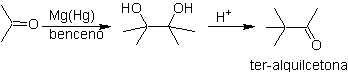
DISCONNECTION OF 1,4-DIOXYGEN COMPOUNDS
Another group of compounds of great importance in chemical synthesis is made up of dioxygenated molecules that are found in a distance ratio of 1.4. These compounds, when subjected to a retrosynthetic disconnection analysis, generate synthons, where one of them, the electrophile or nucleophile, can be considered "anomalous" or "illogical", because the charge assigned to one of the atoms does not can be explained in terms of its intrinsic or induced electronegativity.
1. 1,4-dioxygen compounds (1,4-diO)
In this type of compounds, the disconnection also leads to a logical synthon and to another illogical (non-natural) synthon, which can be a nucleophile or an electrophile, whose synthetic equivalent still has to be adequately reworked, in order to be used in the reaction. chemistry.
1. 1. Compounds 1,4-dicarbonyl
1.1.1. 1,4-diketone compounds
The disconnection alternatives of this type of compounds or molecules to be synthesized (MOb), can lead to the following options:
to. A logical anion synthon and an illogical cation synthon
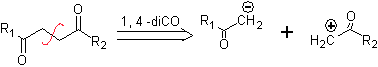
The synthetic equivalent of the anion is the enolate ion or the enol itself of the carbonyl compound. Instead the synthetic equivalent for the carbocation is alpha halocarbonyl. (Umpoloung)
b. A logical cation synthon and an illogical anion synthon
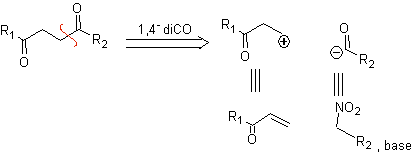
The synthetic equivalent of the logical cation synthon is the α,β–unsaturated carbonyl compound. A suitable synthetic equivalent for the anion synthon may be a nitroalkane anion. The –NO 2 group in alkanes can be transformed into C=O, by means of the Nef reaction, or by the variants of the McMurry reaction, where by action of TiCl 3, the nitroalkane is transformed into an imine, which is then It is hydrolyzed in an acid medium to the respective carbonyl compound.
1.1.2.
1,4-ketoester compounds
γ-Ketoesters, 1,4-diesters, and 1,4-diacids can be disconnected to a natural cation synthon, the synthetic equivalent of which is an α,β-unsaturated carbonyl compound, and to the non-natural (“illogical”) anion synthon. (-) COOR, whose synthetic equivalent is the cyanide ion.
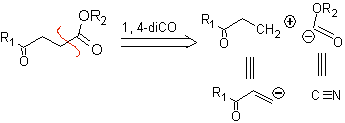
Examples: Propose a synthesis design, from simple and affordable materials, for each of the following molecules:
MOb 35
| MOb 36
| |
MOb 37
| MOb 38
|
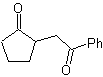
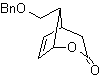
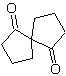
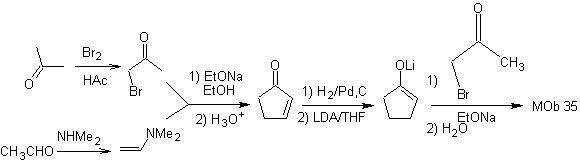
MOb 35 (a). Retrosynthetic analysis . The molecule can be disconnected according to the 1,4-diCO model. The generated precursor cyclopentanone must be previously activated so that its Cα is more nucleophilic, and then be used in the reaction with α.bromoacetone.
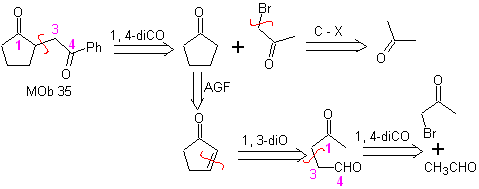 synthesis . The nucleophilicity of cyclopentanone is controlled and guaranteed, using LDA, to arrive at
synthesis . The nucleophilicity of cyclopentanone is controlled and guaranteed, using LDA, to arrive at
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 24406
The 1,6-difunctionalized compounds preferably use the reconnection strategy for their respective synthesis; This strategy can very well be combined with the Diels-Alder reaction, which generally produces six-membered olefinic adducts, or the Birch reduction of benzene rings, which likewise generates six-membered olefinic products.
1.
1,6-dioxygenated compounds
The reaction that generates dicarbonyl compounds, of different possible combinations: diketones, ketoacids, ketoaldehydes, diacids, etc. and at different distances from one another, is undoubtedly the reaction of ozonolysis of olefinic compounds.
Depending on the structure of the substrate and the reaction conditions on the ozonide intermediate formed, an enormous diversity of compounds will be achieved as a result of the cleavage of the olefinic double bond. Of these, those that are in a 1, 6 – dioxygenated ratio are of special interest, as can be inferred from the following synthetic “reconnection” operation:
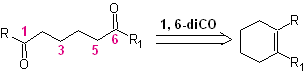
The best way to understand the operation of this "synthetic reconnection operation" is
will be achieved through the solution of the synthesis of the following organic molecules:
MOb 50
| MOb 51
| MOb 52
| ||
MOb 53
| MOb 54
| MOb 55
|
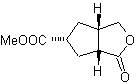
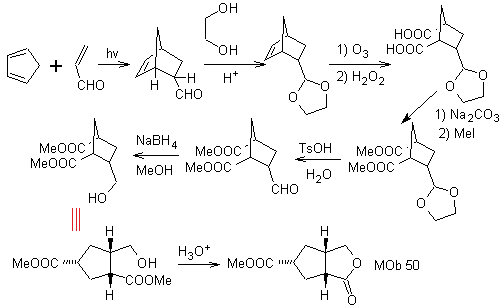
MOb 50 . Retrosynthetic analysis : In the first instance it is disconnected by the lactone function of the molecule. On the generated precursor molecule, in turn, it can be argued that its formation may have occurred from the diacarboxylic acid in position 1-6. Which are reconnected to give rise to the alkene that produced them by oxidative ozonolysis reaction. The alkene formed is a typical Diels-Alder adduct between cyclopentadiene and crotonaldehyde.
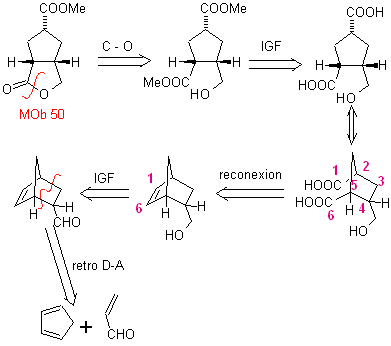
Synthesis: The Diels-Alder reaction between cyplopentadiene and the α,β-unsaturated aldehyde provides the alkene adduct, for its corresponding opening by oxidative ozonolysis, prior to a protection reaction of the aldehyde group, which is subsequently deprotected, to be reduced to the alcohol function. This alcohol reacts with the ester group in an acid medium to form the desired lactone, MOb 50
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 26128
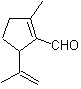
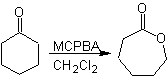
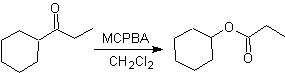
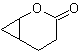
Baeyer–Villiger oxidation
Another reaction that can be associated with the strategy of the Retrosynthesis is the oxidation of ketones by peroxyacids, better known as the Baeyer-Villiger reaction. In cyclic ketones, oxidation with peracids generates lactones. The groups attached to the asymmetric ketones have a migratory ability, which allows, in literal terms, to "insert an oxygen atom" between the carbonyl group and the migrating group, thus producing an ester or a lactone.
It should be taken into account that enones (α, β unsaturated ketones) are not good substrates for the Baeyer-Villiger oxidation, because the alkene is much more reactive than the ketone. However There are special structures where the alkene can be protected by a nearby substituent due to the steric effect and thus direct the attack of the peracid towards the carbonyl group.
| … |
|
Remember that the migratory aptitude of the different groups, in the Baeyer-Villiger reaction, is as follows:
H> Ph> 3º alkyl> cycloalkyl> 2º alkyl> 1º alkyl> Me
Propose a synthesis plan for the following molecules:
MOb 56
L-Dopa | . | MOb 57
| . | MOb 58
|
MOb 59
| MOb 60
| mob 61
|
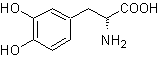
MOb 56. Retrosynthetic analysis.
The alpha amino acid
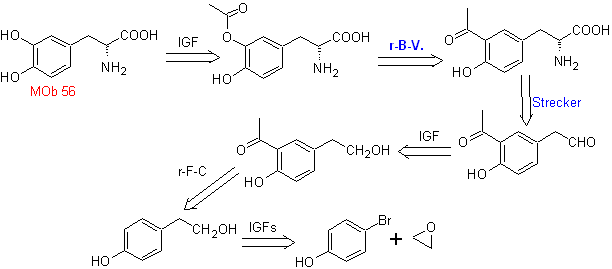
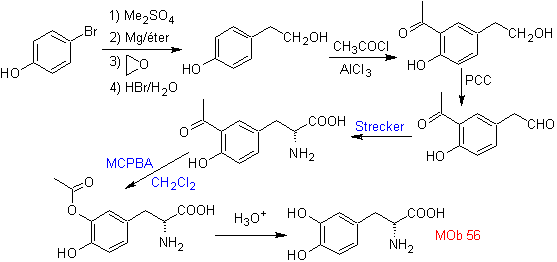
Synthesis. For For the formation of the required Grignard, the ortho OH of the benzene is protected. The Strecker synthesis allows the formation of the alpha amino acid, which is oxidized according to Baeyer-Villiger with a peracid and the product undergoes acid hydrolysis of the ester group, which leads to the formation of
Read more: Baeyer-Villiger oxidation as a strategy in Retrosynthesis
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 29332
The Beckmann transposition as a strategy in
The rearrangement of oximes in an acid medium, called the Beckman rearrangement, produces an amide or lactam if the starting ketone is linear or cyclic, respectively.
To improve the performance of this type of reaction, various catalysts and acid media have been studied. Thus, for example, new acid media used as catalysts, with the indicated purpose, are: TCT/DMF, DAST/CH 2 CL 2 , CF 3 SO 3 H, PCl 5 , HgCl 2 /MeCN and the ZnO.
TCT: trichlorotriazine | …… | DAST: diethylaminosulfide trifluoride |
The migratory fitness of the groups is the same as in the Baeyer-Villiger reaction. Propose a synthesis design for each of the following molecules:
MOb 62 | mob 63 | MOb 64 |
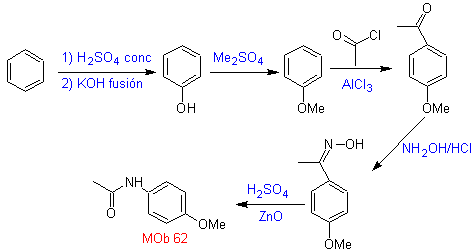
MOb 62 . Retrosynthetic analysis.
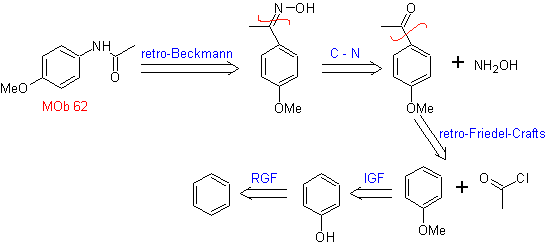
Synthesis. The reaction of the intermediate aromatic ketone with NH 2 OH and its subsequent treatment with an aqueous solution of sulfuric acid and ZnO, allows the formation of the aromatic amide MOb 62.
Read more: Beckmann rearrangement as a strategy in Retrosynthesis
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 21634
Other synthesis strategies that use “illogical” syntons
1.
triple bond addition
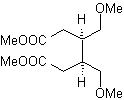
The strategy of "adding" a triple bond, between two oxygenated functions in position 1,4, allows working later with a disconnection based on the chemistry of acetylides. In order to exemplify this strategy, let us see the elaboration of a synthesis plan for
MOb. 40. Retrosynthetic Analysis . A first IGF in
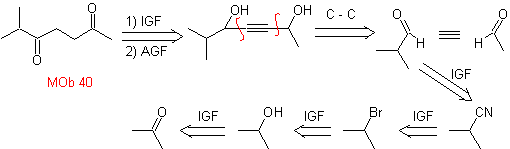
Synthesis. The diacetylide or acetylide in stages, combines with molecules of different aldehydes, the intermediate molecule formed is hydrogenated and then its alcohols are oxidized to the diketonic compound Mob 40.
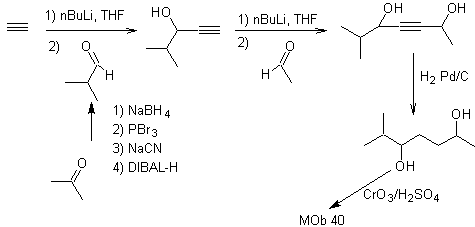
The γ-lactones can also be prepared in an analogous way, as shown below:

2.
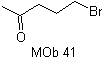
Adding the COOR group as an activating group
The addition of the COOR group, in addition to activating the anion synthon, facilitates the disconnection of a 1,4 diX molecule.
synthesize |
|
MOb 41. Retrosynthetic analysis.
Read more: Other synthesis strategies that use "illogical" syntons
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 51177
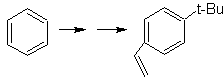
SYNTHESIS OF AROMATIC COMPOUNDS I
(Synthesis Tree Method)
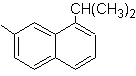
Propose a synthesis plan, using toluene or xylene as starting materials, for the following molecules:
(MOb 12)
1-isopropyl-7-methylnaphthalene | (MOb 13)
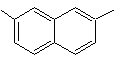
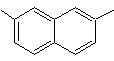
2,7-dimethylnaphthalene |
(MOb 14)
1-isopropyl-6-methylnaphthalene | (MOb 15)
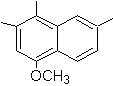
4-methox-1,2,7-trimethylnaphthalene |
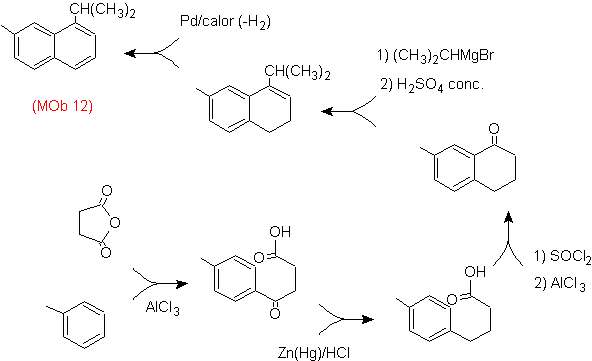
Solution: (MOb 12).
In the strategy that is assumed, it is taken into account that the last stage can respond to an "aromatization" process, for which it is proposed that the precursor molecule presents a non-aromatic ring, with a double bond.
on the carbon containing the double bond and the alkyl group.
This structure can be achieved by the action of a Grignard on a carbonyl and the subsequent dehydration of the alcohol formed. The ketone is formed by acylation on the appropriate benzene compound with succinic anhydride and its subsequent Friedel-Crafts intramolecular acylation closures.
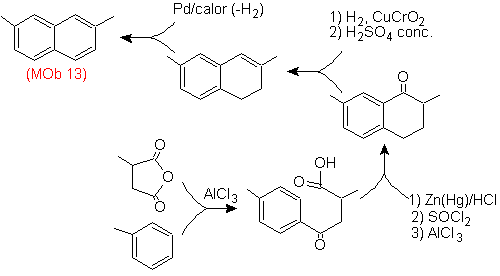
Solution (MOb 13).
Once again, the precursor molecule has to be "aromatized", the most appropriate strategy among others It is based on the combination of acylation with substituted succinic anhydride and the Clemmensen reduction.
The final carbonyl is reduced to alcohol that will then be dehydrated with molecular hydrogen and a catalyst called copper chromyl.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 29605
SYNTHESIS OF AROMATIC COMPOUNDS II
(Synthesis Tree Method)
Although one of the first problems to be solved in the synthesis of multi-substituted aromatic compounds is the control of the orientation effects and the formation of undesired isomers, it is also important to study the reactivity of the arenes, since at some point In the sense of the presence of aliphatic groups in the aromatic compound, many times, they present characteristics and reactivities, typical of the type of organic compound to which they belong and the particular ones that result from the mutual interaction of the aliphatic and aromatic groups.
On this purpose, it is based, the synthesis of molecules No. 20 to 27, this time from specified materials, so the question is presented as follows: What are the reactions that Justify the following transformations?
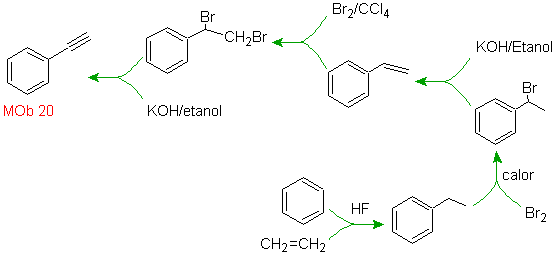
Mob 20 solution.
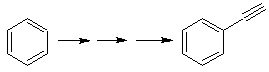
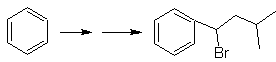
We know that there is no possibility of the acetylide ion acting directly on benzene, therefore the triple bond is obtained from an alkyl group
vec-dibrominated, which is obtained by brominating styrene, previously obtained by dehydrobrominating of a benzyl halide formed by a bromination by the free radical mechanism on ethylbenzene
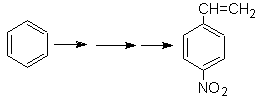
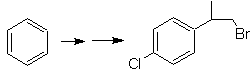
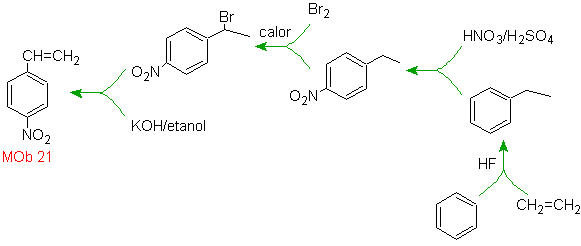
Solution Mob 21.
The para nitrostyrene cannot be obtained by direct nitration of styrene, because the ethenyl group attached to the ring is unstable under nitration conditions.
As such, the precursor molecule will have a group that is easy to dehydrobrominate. This precursor is obtained by radical bromination of the ethyl group linked to the benzene ring, which was previously nitrated mainly in the para position.
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 32812
SYNTHESIS OF ALCOHOLS
(Synthesis Tree Method)
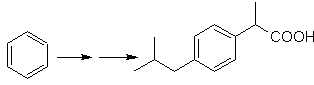
Propose a synthesis plan for the indicated target molecules from the indicated single molecules (MOb 30 -41). To do this, use the reagents and reaction conditions that you think are necessary:
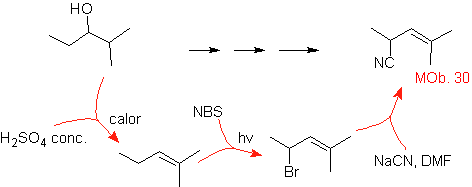
MOb 30 solution.
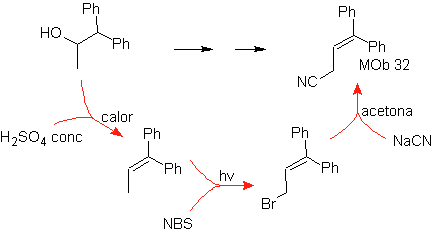
Strategy: It is observed that the starting molecule has been dehydrated and in the initially unsubstituted allylic position, a hydrogen has been displaced by the cyano or nitrile group. This last reaction can occur only if the precursor molecule is an allylic halide, which is why it is proposed as a precursor of
The Br is introduced in the desired position with the NBS and the alkene is the product of the dehydration of the starting molecule.
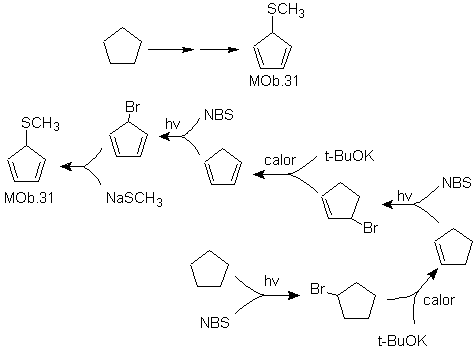
Solution MOb 31.
Strategy : It is a uncle ether, the necessary precursor molecule will be a 1,3-cyclopentadiene halide.
This halide is prepared by the action of NBS on the dienic cycloalkene, which in turn is prepared by the dehydrobromination of the precursor molecule, which is reached by the action of NBS on the cycloalkene formed. previously by dehydrohalogenation of the starting molecule brominated by radicals
Mob 32 solution.
Strategy : It is similar to the one used in obtaining
- Details
- Wilbertrivera
- ORGANIC SYNTHESIS
- Hits: 25509
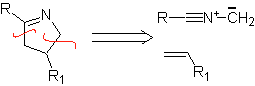
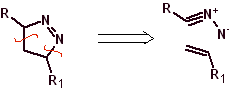
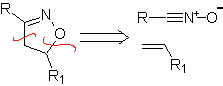
These reactions normally form five-membered heterocyclic rings, for which the reaction between a dipolar n1,3 compound and an alkene is necessary. The reaction is a [3-2] cycloaddition. The 1,3-dipolar compounds that have had the most use to form pentagonal heterocycles are:
 |  |
 | 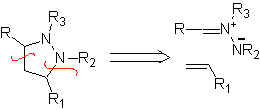 |
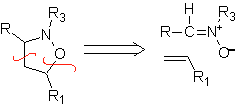 | 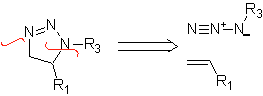 |
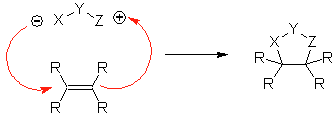
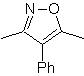
| MOb: 86
| . | MOb: 87 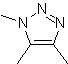 | .. | MOb: 88 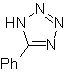 |
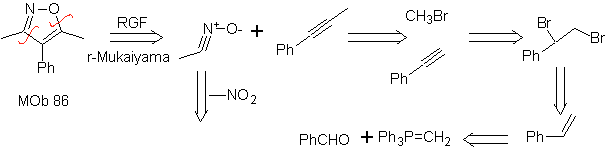
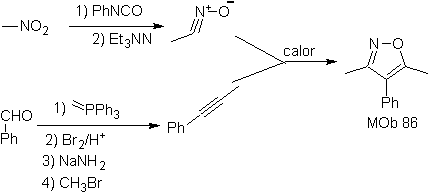
Read more: Synthesis of heterocycles by intermolecular cyclization