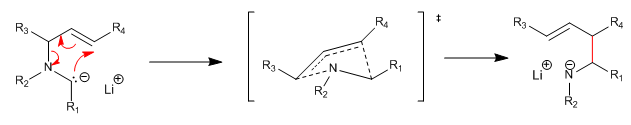
Reaction analogous to the Wittig rearrangement, in which alpha metal ethers rearrange alkoxides, generating secondary or tertiary alcohols after hydrolysis. In the case of aza-[2,3]-Wittig, these are alpha metallated allylic tertiary amines which produce secondary amines after hydrolysis.

The mechanism of aza-[2,3]-Wittig proceeds through a concerted process, with a five-membered, six-electron envelope-shaped cyclic transition state.