AMINO ACIDS AND PEPTIDES THEORY
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 56290
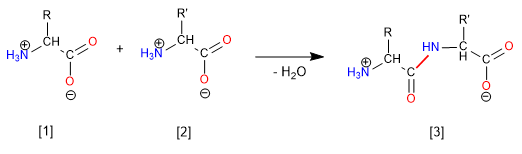
Amino acids are organic compounds that contain carboxylic acid and amino functions. Two amino acids can be joined under suitable conditions, through an amide bond, forming a dipeptide. The dipeptide can incorporate a third amino acid, forming a tripeptide. Chains with more than 50 amino acids are called proteins.
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 187048
The 20 amino acids that make up proteins are: Serine (Ser,S), Threonine (Thr,T), Cysteine (Cys,C), Asparagine (Asn,N), Glutamine (Gln,Q) and Tyrosine (Tyr, Y), Glycine (Gly,G), Alanine (Ala,A), Valine (Val,V), Leucine (Leu,L), Isoleucine (Ile,I), Methionine (Met,M), Proline (Pro,P ), Phenylalanine (Phe,F) and Tryptophan (Trp,W), Aspartic Acid (Asp,D) and Glutamic Acid (Glu,E), Lysine (Lys,K), Arginine (Arg,R) and Histidine (His, h)
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 146997
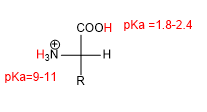 The presence of acid (-COOH) and basic (-NH 2 ) groups gives amino acids characteristic acid-base properties.
The presence of acid (-COOH) and basic (-NH 2 ) groups gives amino acids characteristic acid-base properties.
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 56450
Amino acids can be obtained by halogenation of carboxylic acids, followed by nucleophilic substitution with ammonia. The halogenation of the a position of the carboxylic acid is carried out with the Hell-Volhard-Zelinsky reaction.
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 46214
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 32562
This is a widely used method for preparing amino acids in the laboratory. Ethanamide amidate is reacted with the halogenated malonium ester , obtaining the compound which is alkylated and hydrolyzed to give a diacid , which decarboxylates to form the amino acid .
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 54064
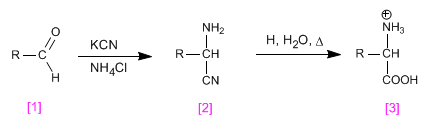
The Strecker synthesis allows obtaining amino acids from aldehydes or ketones.
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 30187
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 30265
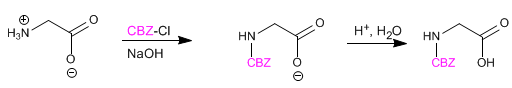
The amino group is protected to prevent its reaction with the acid group of the second amino acid. Once the peptide bond is formed, deprotection is performed, again leaving the amino group free.
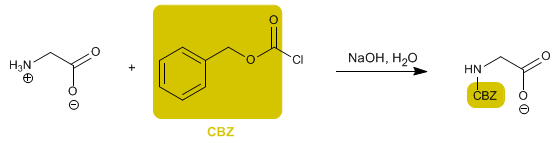
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 23181
The acid group is protected by transforming it into an ester by reaction with an alcohol. Methyl, ethyl or tert -butyl esters are prepared by esterification and hydrolyzed (deprotected) in a basic medium.
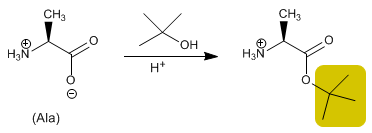
- Details
- Germán Fernández
- AMINO ACIDS AND PEPTIDES THEORY
- Hits: 48417
The synthesis of the dipeptide Glycine-Alanine (Gly-Ala) takes place through the formation of the amide bond (peptide) between the acid group of Glycine and the amino group of alanine. Therefore, it is necessary to protect the amino group of Glycine and the acid of alanine.