ENOLATE ALKYLATION
- Details
- Germán Fernández
- ENOLATE ALKYLATION
- Hits: 731
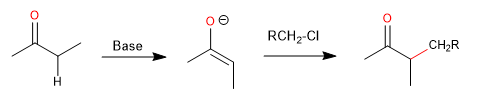
The formation of carbon-carbon bonds is essential for the construction of the molecular framework of organic molecules through synthesis. A fundamental process for this bond formation is the reaction between a nucleophilic carbon and an electrophilic carbon.
From a mechanistic point of view, these reactions are usually SN2 reactions, where the carbon nucleophile displaces a halide or other leaving group with inversion of the configuration in the alkyl group. Efficient carbon-carbon bond formation requires that SN2 alkylation be the major reaction. We need to consider the following factors: (a) the conditions for generating the carbon nucleophile; (b) the effect of reaction conditions on the structure and reactivity of the nucleophile; and (c) the regio- and stereoselectivity of the alkylation reaction. The reaction can be used with various carbonyl compounds, such as ketones, esters, and amides.
- Details
- Germán Fernández
- ENOLATE ALKYLATION
- Hits: 1916
This chapter addresses the fundamental aspects of the structure and stability of carbanions, focusing on those stabilized by carbonyl substituents and other electron-withdrawing groups (EWGs). A relationship is established between the properties and reactivity of these carbanions and their application as nucleophiles in synthesis. The acidity of C-H groups, determined by the stabilizing functional group, is fundamentally linked to the formation of enolates, highlighting the relationship between kinetic or thermodynamic control in enolate formation by deprotonation.
Control of the equilibrium between enolate and its conjugate acid is based on the choice of base. The reaction can be carried out under conditions where the enolate is in equilibrium with its conjugate acid or where the reactant is completely converted to its conjugate base. The amount and strength of the base are key determinants. Current procedures for enolate alkylation typically involve complete conversion to the conjugate base, allowing for greater regiochemical and stereochemical control. The solvent and other coordinating additives also significantly affect the structure and reactivity of carbanions generated by deprotonation.
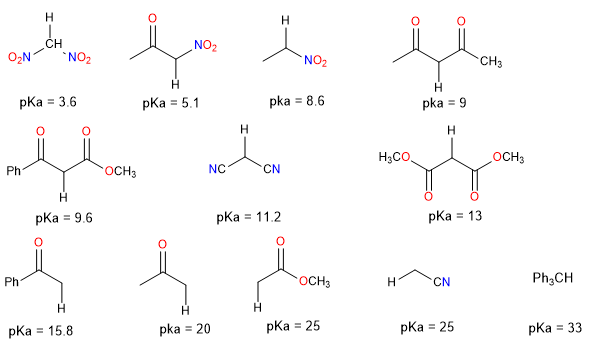
The following table provides approximate pK data for various functional groups and some commonly used bases.
Compounds with two charge-stabilizing groups.
- Details
- Germán Fernández
- ENOLATE ALKYLATION
- Hits: 3068
Deprotonation of carbonyl compounds is an important method for generating enolates. An asymmetric dialkyl ketone can form two regioisomeric enolates in the deprotonation, and it is essential to know the conditions under which one of them is obtained predominantly.
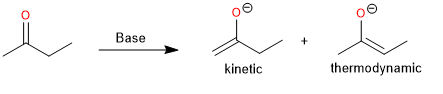
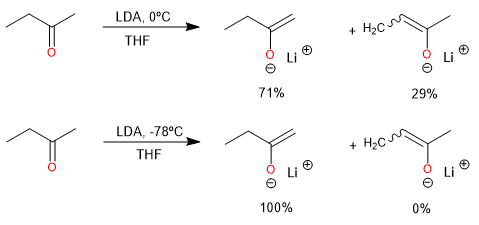
Although it may not be possible to direct the deprotonation to form exclusively one enolate instead of the other, experimental conditions can often be chosen that favor one of the regioisomers. The composition of a mixture of enolates can be governed by kinetic or thermodynamic factors. Kinetic control is exerted when the composition of the product is determined by the relative rates of the competing proton abstraction reactions. By choosing the experimental conditions appropriately, it is possible to establish both kinetic and thermodynamic control.
The conditions for kinetic control of enolate formation are those in which the deprotonation is rapid, quantitative, and irreversible.
Using a very strong base such as LDA or LiHMDS in an aprotic solvent.
Absence of excess ketone.
Lithium is a better counterion than sodium or potassium for the regioselective generation of the kinetic enolate, as it maintains a tighter coordination to the oxygen and reduces the rate of proton exchange.
The use of an aprotic solvent is essential because protic solvents allow equilibration of the enolate by reversible protonation-deprotonation, leading to the thermodynamically controlled enolate composition. Excess ketone also catalyzes equilibration by proton exchange.
Read more: Regioselectivity and Stereoselectivity in the Alkylation of Enolates