In 1855, Wurtz treated alkyl halides with sodium metal to obtain the corresponding symmetrical alkane. The coupling of two sp 3 carbons belonging to alkyl or aryl halides by treatment with sodium metal is known as Wurtz synthesis. On the other hand, the coupling of an alkyl halide with an aryl halide is called a Wurtz-Fittig reaction.
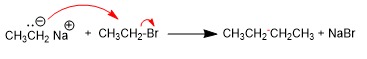
1. Homocoupling of bromoethane to yield butane
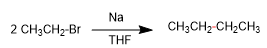
2. Cross coupling of bromoethane and bromopropane to yield pentane. The yield drops due to the formation of homocoupling products.
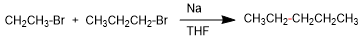
3. Wurtz-Fittig coupling between p-bromoanisole and bromoethane.
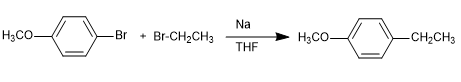
Mechanism:
The currently accepted mechanism for the Wurtz reaction occurs in two stages:
Stage 1. Formation of a carboanion by metal-halogen exchange

Stage 2. Nucleophilic attack of the carboanion on the alkyl halide by SN2 mechanism.