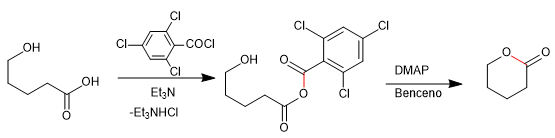
In 1979, Yamaguchi et al. developed a procedure to obtain esters and lactones under mild conditions through alcoholysis of the corresponding mixed anhydride.
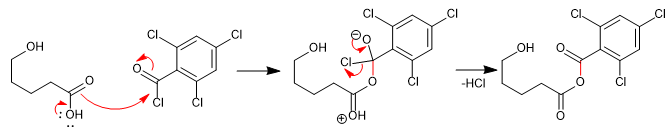
The formation of a lactone starts from the treatment of the hydroxy acid with 2,4,6-trichlorobenzoyl chloride in the presence of triethylamine, necessary to neutralize the hydrochloric acid released at this stage. The mixed anhydride produced is treated with DMAP in toluene, under high dilution conditions, yielding the desired lactone.
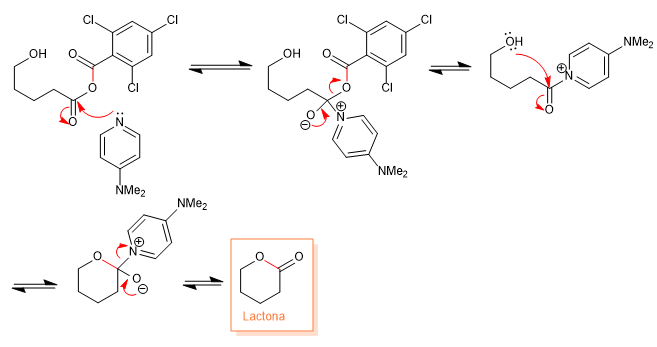
Mechanism:
1. Formation of the mixed anhydride
2. Intramolecular esterification catalyzed by DMAP