There are several criteria to determine the degree of aromaticity of a heterocycle:
A) Link lengths.
A heterocycle is all the more aromatic the smaller the difference between the lengths of the different bonds that make up the ring. Benzene has the same length in all its carbon-carbon bonds, which makes it the most aromatic compound. The heterocycles present differences in the bond distances, being less aromatic the greater these differences are.
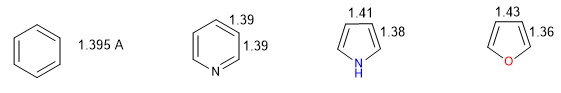
All C-C bonds in benzene measure 1.395 A, an intermediate bond distance between single (1.48 A) and double (1.34 A) bonds. In furan there is a notable difference between the length of the bonds, indicating a lower aromaticity than in pyrrole. Pyridine, on the other hand, has very similar C-C bond distances between them and similar to those of benzene, which shows greater aromaticity than pyrrole or furan.
B) Nuclear magnetic resonance data.
Another measure of the degree of aromaticity consists in comparing the coupling constants of the different hydrogens. If the bond distances between carbons are the same, the coupling constants between the hydrogens attached to them are also the same. Aromatic systems present a chemical shift between 7 and 8.5 ppm due to the phenomenon of magnetic anisotropy.
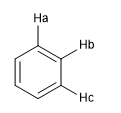
Jab = Jbc if the distances Ca - Cb and Cb - Cc are equal, which shows a highly aromatic system.
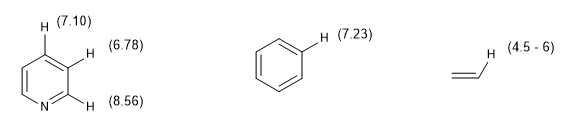
The chemical shift of aromatic hydrogens makes it easy to distinguish them from vinyl hydrogens.
C) Resonance energies.
REPE (resonance energy per electron p) data is used to compare the degree of aromaticity of different heterocycles.
The REPE is obtained by dividing the resonance energy of a system by the number of pi electrons it possesses. We can define the resonance energy as the energy difference between the molecule with localized and delocalized bonds.
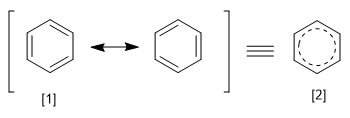
[1] Canonical structure
[2] Resonance hybrid
| Compound | REPE |
| Benzene Pyridine Pyrimidine Pyrrole Thiophene Furan |
0.065 0.058 0.049 0.039 0.032 0.007 |
The more electronegative the heteroatom is, the less aromatic the heterocycle presents, since the pi electrons are attracted to it and conjugation decreases. This factor explains the higher aromaticity of pyrrole compared to furan. In heterocycles with heteroatoms of similar electronegativity, but in which one heteroatom has d orbitals, the one without d orbitals presents greater aromaticity. D orbitals, being more diffuse, give less effective overlaps with carbon atoms and impair conjugation. This factor explains the higher aromaticity of pyrrole compared to thiophene.