Their objective is to determine the positions by which both cycles condense. The base heterocycle is literate, each bond is assigned a letter, starting from the bond that joins atoms 1,2, to which the letter a is associated, until reaching the condensation bond, whose letter appears inside the bracket in the heterocycle name. The attached heterocycle is numbered from atom 1 to the condensation carbons, by the shortest path. The numbers of these condensing carbons are entered in the square bracket next to the literal letter. [1,2-b] indicates that the fusion bond contains the 1,2 carbons of the appended heterocycle and the b bond of the base.
Let's see an example.
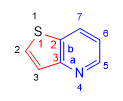
Base: Pyridine (Rule 1. component with nitrogen)
Annex: Thiophene (thiene)
Annexed numbering: starts at the sulfur and goes towards the C. bridge.
Base literation: it begins in the nitrogen and goes towards the C. bridge.
Peripheral numbering: Start at sulfur to give heteroatoms the lowest locants. (rule 1)
Name: Thieno[3,2-b]pyridine
This condensed heterocycle has pyridine as its base component (rule 1) which is literally assigned the letter "a" to the nitrogen-carbon bond (1-2) in the direction of the condensation carbons. The following link 2-3 is literalized as "b" and it is the one that indicates the condensation position of both heterociles and we will use it to build the name.
We now move on to numbering the attached component. We begin by assigning locant 1 to the heteroatom (sulfur) and continue the numbering towards the condensation carbons, which take locants 2,3.
Once the base has been written and the annex numbered, we indicate the condensation position of both heterocycles with the notation [3,2-b].
Finally, the name is built starting with the attached heterocycle, followed by the condensation position and finally the base is named: Thieno[3,2-b]pyridine.
Note that the numeric locators are placed in the order indicated by the literation: a-->b, 3-->2