Let us now consider the cases of unsaturated heterocycles (with at least one double bond), but which do not have the maximum number of double bonds. They are named as if they were completely unsaturated, putting the dihydro, tetrahydro, hexahydro, etc. particles before the name. (always indicative of an even number of hydrogens, as corresponds to formal hydrogenations of one, two, three, etc. double bonds). Keep in mind that formal hydrogenations may have occurred on neighboring or remote carbon atoms in the heterocycle.
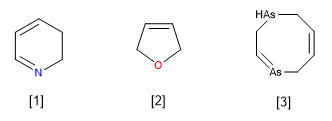
A pyridine heterocycle missing a double bond at the 2,3-position. (2,3-Dihydropyridine)
Furan heterocycle missing a double bond. (2,5-dihydrofuran).
![]() Note that dihydros can be used with non-adjacent carbons.
Note that dihydros can be used with non-adjacent carbons.
Diarsocine heterocycle missing two double bonds. (1,2,5,8-Tetrahydro-1,4-diarsocine)