In this section we are going to study the influence of annular stresses on the properties of heterocycles.
A) Angular stress in small heterocycles (3 and 4 members).
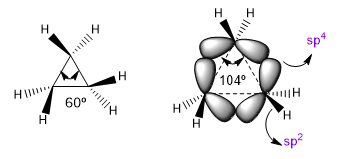
The natural bond angles of a sp3 carbon are 109.5º, however in three-membered cycles this angle decreases to 60o, which produces enormous tension. To alleviate this tension, the bonds between carbons are no longer straight, bending, giving rise to curved bonds called "banana bonds". The angles formed by these bonds are 104º, a more tolerable discrepancy.
| Hybridization | Bond angle |
| sp sp2 sp3 sp4 |
180º 120º 109.5º 104º |
This situation makes CC bonds tend to a theoretical sp4 hybridization while C-H bonds have sp2. These hybridizations will allow us to explain variations in spectroscopic and basicity properties. In the infrared spectra it is observed that as the p character of a bond decreases, the absorption moves towards a greater number of waves. Let's compare non-cyclic amides with stressed lactams to see this effect.
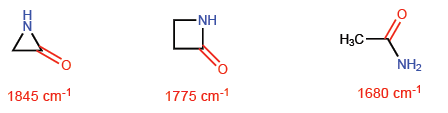
In azetidin-2-one, the p-character of the C=O bond is smaller than in the other molecules and therefore the absorption of infrared radiation moves towards a greater number of waves.
Another property that is influenced by the strain of these heterocycles is the basicity. An increase in the p character of the lone pair produces a decrease in basicity. Thus, between a cyclic and an acyclic amine, the cyclic is less basic.
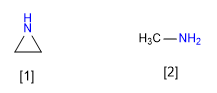
[1] Aziridine
[2] Methylamine
Aziridine is less basic than methylamine. The lone pair hybridization of aziridine approximates sp2 while that of methylamine is sp3 . The smaller p character of aziridine makes it less basic.
B) Annular stress in large rings. Cyclooctin is known to be the smallest cyclic alkyne in existence. The smallest cycloalkynes produce a distortion in the bond angle corresponding to sp hybridization, generating a tension in the molecule that makes it unfeasible.
However, there is an exception to this rule observed in thiacycloheptines, which is explained by the greater length of the C-S bond compared to the C-C.
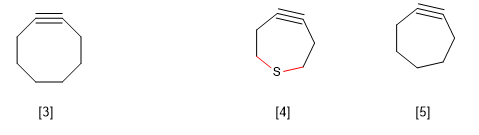
[3] Cyclooctine
[4] The greater length of the CS bonds compared to the CC allows the existence of this cyclic alkyne.
[5] Does not exist
C) Stiffness of bicycles.
The rigidity of the bicilli, the impossibility of turning, prevents some resonances, giving rise to anomalous properties in the molecule. Amides delocalize lone pairs from nitrogen to carbonyl oxygen, which removes the basicity of nitrogen. However, when the amide is part of a bicyclic system, this assignment is not viable and the nitrogen appears strongly basic.
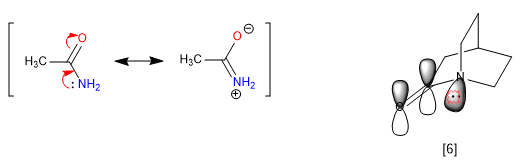
[6] The nitrogen pair cannot be transferred, because its orbital is not parallel to that of the carbonyl.
The 1-azabicyclo[2.2.2]octane nitrogen presents a remarkable basicity comparable to aromatic amines, with a pKa for its conjugate acid of 5.3. The spectroscopic data for this amide are also unique. Thus the carbonyl absorbs at 1762 cm -1 , more typical of a stressed ketone than an amide. The explanation lies once again in the impossibility of transferring the free pair of nitrogen.
D) Conformational equilibrium in sis-membered rings .
The shorter bond length C-O compared to CC introduces certain peculiarities in the conformational equilibria of six-membered heterocycles with oxygen.
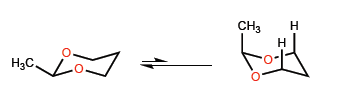
The short C-O distances cause the methyl to move closer to the 3-position hydrogens, giving rise to 1,3-diaxial interactions that shift the conformational equilibrium all the way to the left.
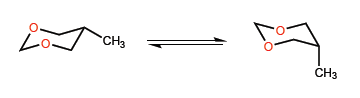
In this second example, the situation changes, since the interaction between methyl and oxygen lone pairs is not very important, and a tert-butyl group can even be located in an axial position.