The reaction of carbonyls with thiols forms thioacetals that can be used as protecting groups or to reduce the carbonyl to an alkane.
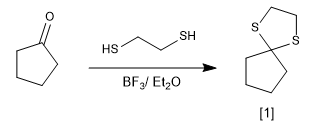
thioacetal
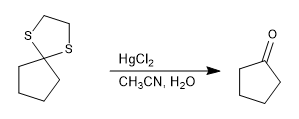
Thioacetal is stable in acid media but can be hydrolyzed with mercury(II) salts.
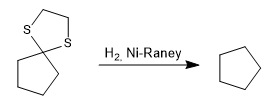
Reduction of the thioacetal to an alkane by catalytic hydrogenation with Ni-Raney is also possible.
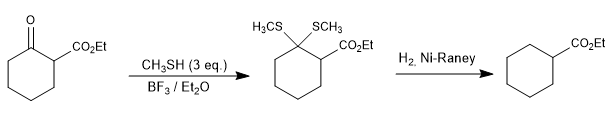
In the following example, the ketone is selectively reduced in the presence of the ester using the thioacetals.