The 3-keto esters or β -keto esters are obtained by the Claisen condensation of two esters.
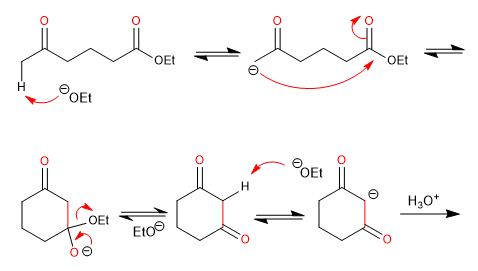
Diesters produce cyclic 3-ketoesters by intramolecular Claisen condensation, also called Dieckmann condensation
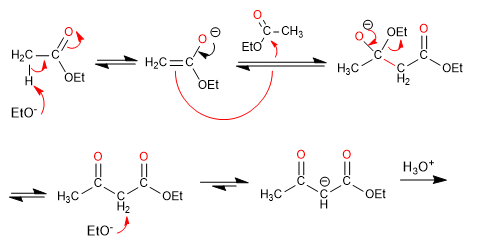
Mixed Claisen produces 1,3-dicarbonyl compounds by condensation between ketone and ester.
- Obtaining 3-ketoesters
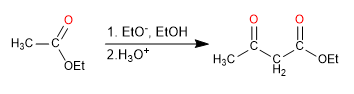
Mechanism:
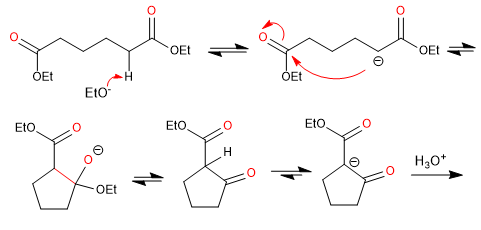
- Obtaining cyclic 3-ketoesters
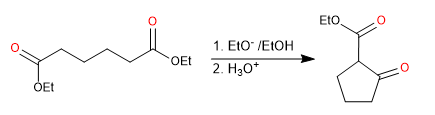
Mechanism:
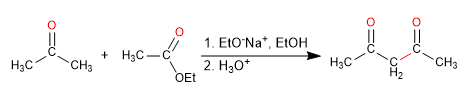
- Obtaining 1,3-dicarbonyls.
The mixed Claisen condensation between ester and ketone produces 1,3-dicarbonyl compounds. Aldehydes are not effective in this synthesis as they react via aldol condensation.
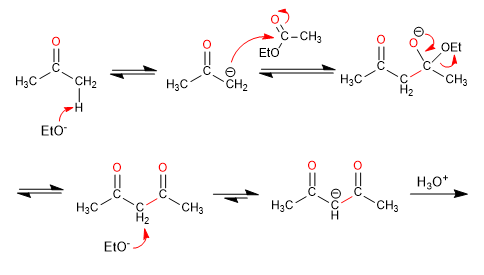
Mechanism:
- Obtaining cyclic 1,3-diketones.
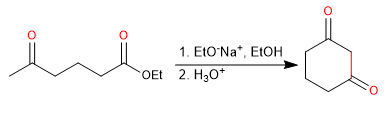
Mechanism: