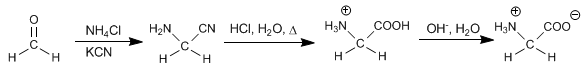The Strecker synthesis allows obtaining amino acids from aldehydes or ketones.
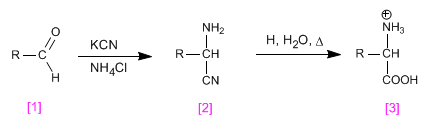
aldehyde reacts with ammonia in an acid medium to form an imine, which adds cyanide, forming a-aminonitrile , which is hydrolyzed to carboxylic acid in the last stage.
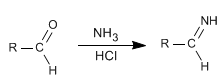
Mechanism of the first step:
Stage 1 . imine formation
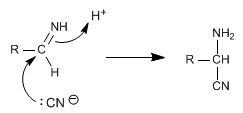
Stage 2 . cyanide addition
Subsequent hydrolysis of the nitrile yields the amino acid.
Starting from methanal, the amino acid glycine is obtained.