a) Amides can be obtained by reacting amines with alkanoyl halides and anhydrides .
Ethanoyl chloride reacts with two equivalents of methylamine to form ethanamide.
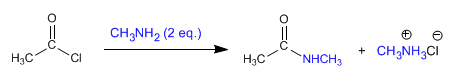
The second equivalent of amine is used to collect the hydrochloric acid and favor the equilibria.
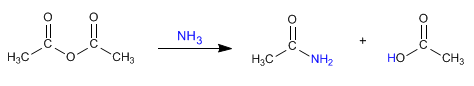
Ethanoic anhydride reacts with ammonia to form ethanamine and ethanoic acid.
b) Carboxylic acids react with ammonia and amines to form amides .
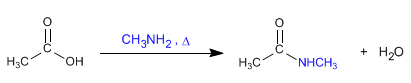
c) The reaction of ammonia and amines with esters forms amides.
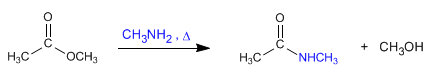
d) Preparation of urea.
The reaction of ammonia with carbon dioxide, followed by heating under pressure, generates urea. The reaction proceeds in the following stages.
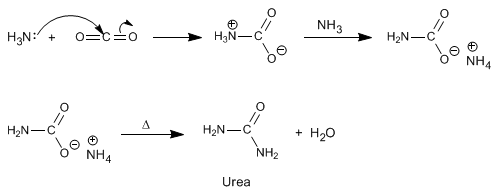
In the world, large quantities of urea are produced by this method, to be used as fertilizer.