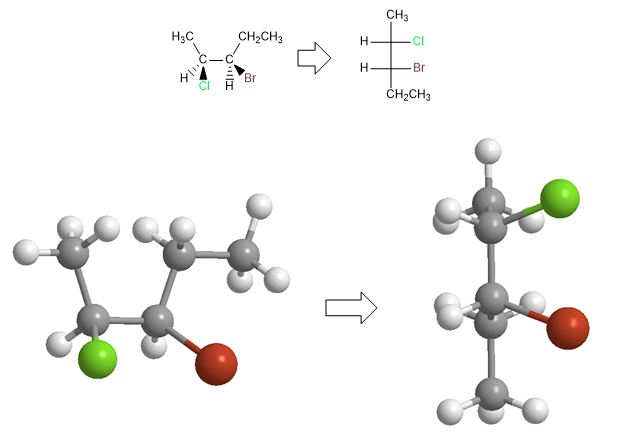
Projecting consists of drawing a molecule in two dimensions (plane). In the Fischer projection, the molecule is drawn in the shape of a cross with the substituents that go to the bottom of the plane in the vertical and the groups that come out towards us in the horizontal, the point of intersection of both lines represents the projected carbon.
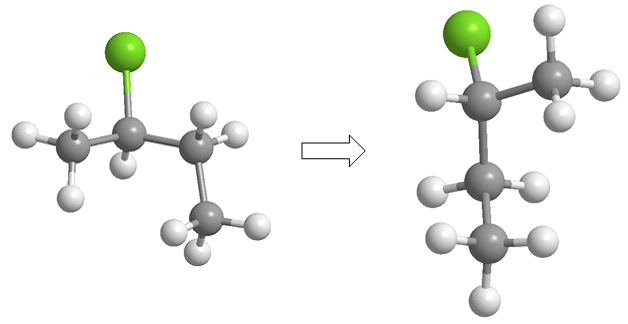
Although it is customary to leave the carbon chain vertical, the molecule can be rotated in different ways, giving rise to Fischer projections that appear to be different but actually represent the same molecule.
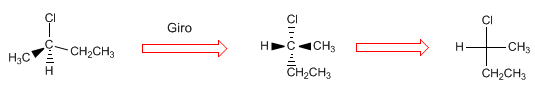
To check that the projection is correct, we are going to give R/S notation to the molecule and its projection.
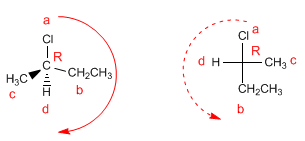
Note that with hydrogen on the horizontal, the spin gives the changed notation.
Now we will make the projection of a molecule with two chiral centers
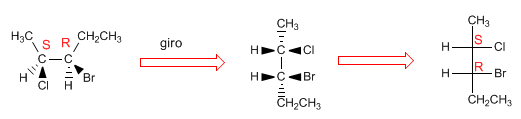
To project a molecule in Fischer it is necessary to draw it in the eclipsed conformation. The substituents that remain in the plane are placed above and below the projection. The groups that come out towards us (wedges) are arranged to the right in the projection, and those that go to the bottom (dashed lines) are arranged to the left.