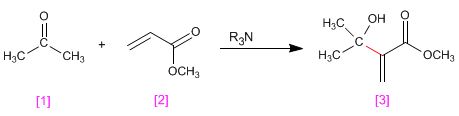
Aldehydes and ketones [1] react with a,b -unsaturated compounds [2] in the presence of tertiary amines that act as catalysts, to form a -hydroxyalkylated products [3] .
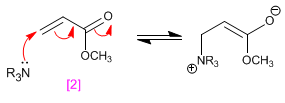
The mechanism occurs with the attack of the tertiary amine on the carbon b of the a,b -unsaturated, forming an enolate that attacks the carbonyl. In a last stage, the elimination of the amine occurs.
Stage 1. Addition to carbon b of the amine to form an enolate
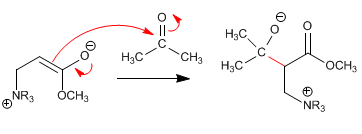
Stage 2. Nucleophilic attack on the carbonyl
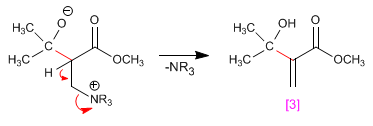
Step 3. Elimination of the amine
References:
1. D. Basavaiah, PD Rao, RS Hyma, Tetrahedron 1996, 52, 8001–8062.
2. E. Ciganek, Org. react. 1997, 51, 201–350.









