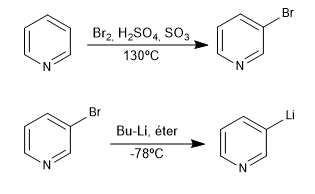
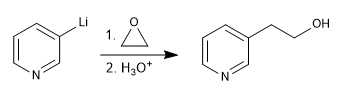
This reaction forms organolithiums from a halogenated pyridine. Organolithics allow the attack of very varied carbon electrophiles, such as: primary alkyl halides, aldehydes, ketones, nitriles, esters, epoxides...
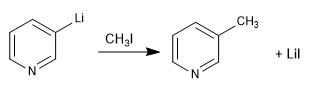
a) Alkylation of pyridinyl lithium
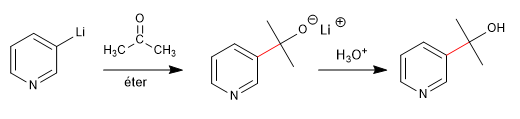
b) Reaction with aldehydes and ketones
c) Opening of oxacyclopropanes
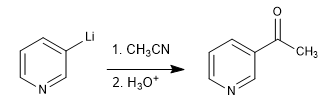
d) Reaction with nitriles