Pyridines with 2,4-alkyl groups have acidic hydrogens in the neighboring position of the ring. These hydrogens can be removed by using strong bases such as tert-butyllithium, LDA, etc.
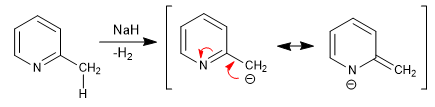
The base obtained has an important nucleophilic character, which allows the chain to be lengthened by attacking different electrophiles.
.
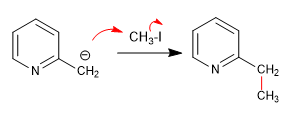
a) Attack on primary haloalkanes
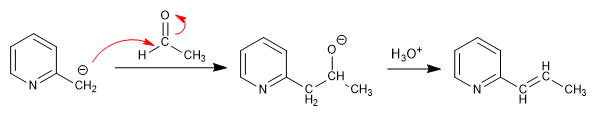
b) Attack on carbonyls
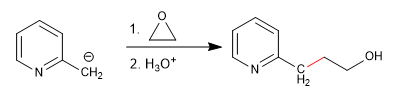
c) Attack on oxacyclopropanes