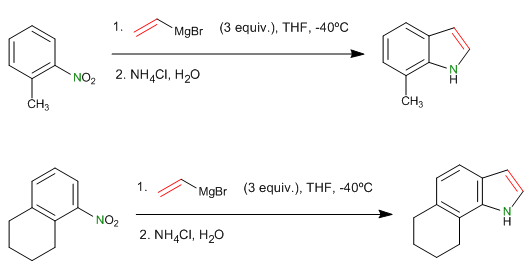
In 1989, G. Bartoli described the reaction of substituted nitroarenes with Grignard vinyl organometallics at low temperature, yielding substituted indoles after aqueous work-up. Three equivalents of magnesium are necessary, added to a cold nitroarene solution, which is stirred for twenty minutes, followed by the addition of saturated ammonium chloride solution and final extraction with ether. Better performance is obtained with indoles substituted in the ortho position to the nitro.
Mechanism:
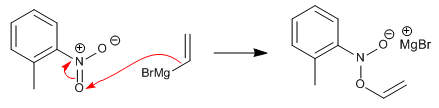
Step 1. Addition of the Grignard reagent to the oxygen atom of the nitro group
Stage 2. Decomposition of the O-alkenylated product, forming a nitrosoarene.
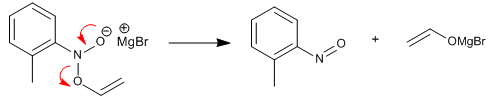
Stage 3. Nucleophilic attack of the second equivalent of the Grignard reagent on the oxygen of the nitroso compound
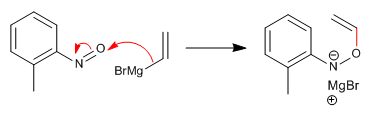
Stage 4. Sigmatropic regrouping [3,3]
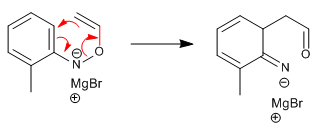
Step 5. Intramolecular cyclization
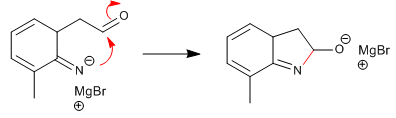
Stage 6. Rearomatization of the carbocycle
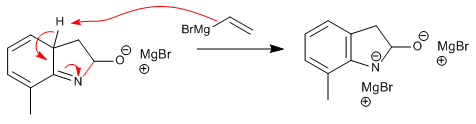
Stage 6. Aqueous treatment
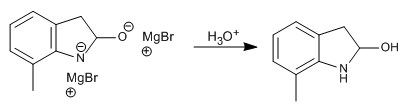
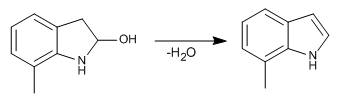
Stage 7. Dehydration