asymmetric organocatalysis
Introduction
Despite the importance of chirality, obtaining chiral molecules in enantiomerically pure form has remained extremely limited until very recently. Since the end of the 19th century, the synthesis of chiral molecules in a stereoselective manner has been a synthetic challenge of great magnitude to which organic chemists have responded with great ingenuity and brilliance.
In broad lines, three basic strategies can be considered for obtaining enantiomerically pure compounds, such as
a) the resolution of racemates,
b) the use of natural optically active molecules
c) asymmetric synthesis.
Use of chiral auxiliaries:
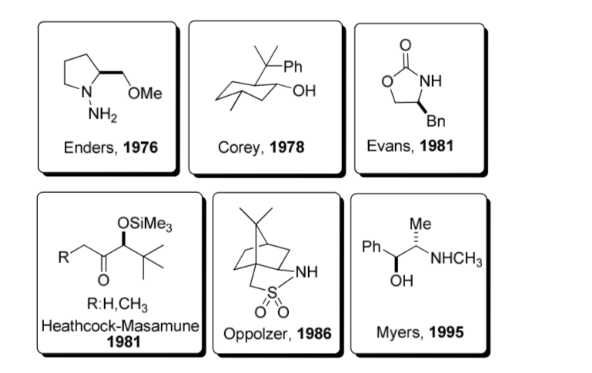
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically, with the selective formation of one of two enantiomers.
This strategy gained considerable popularity in the 1980s and today a wide range of auxiliaries is known for a large number of reactions. Some of the most representative auxiliaries are shown in the figure
What is asymmetric organocatalysis?
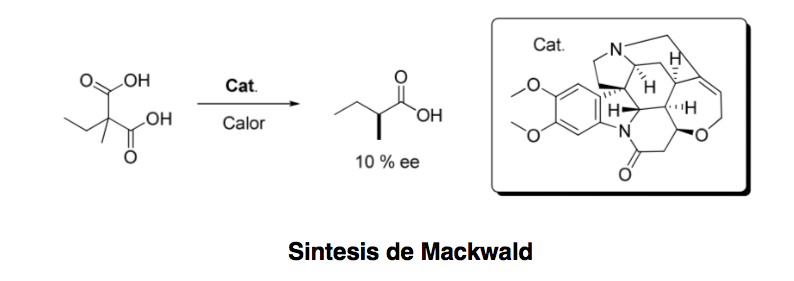
Asymmetric organocatalysis or asymmetric catalysis can be defined as "the acceleration of chemical reactions with a substoichiometric amount of an organic compound, which does not contain any metal atoms using organic molecules of a chiral nature." Although the first example of an organocatalytic enantioselective transformation Dating back to 1904, when Mackwald carried out the decarboxylation of an acid derivative in the presence of brucine, it has not been until a few years ago that this field has experienced a spectacular revival.
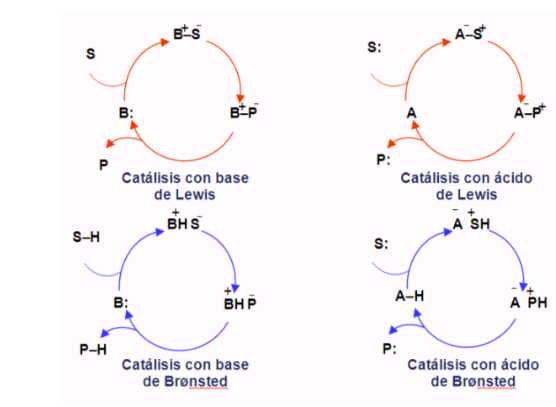
Organocatalytic cycles
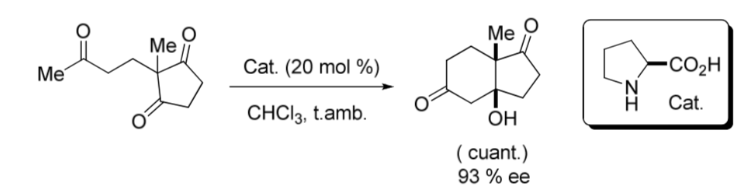
An important milestone in the area of asymmetric organocatalysis was the intramolecular aldol reaction catalyzed by L -Proline published in the late 1960s and early 1970s by the Hajos and Parrish groups; and by another Ender, Wiechert and Sauer regardless as shown in the following image:
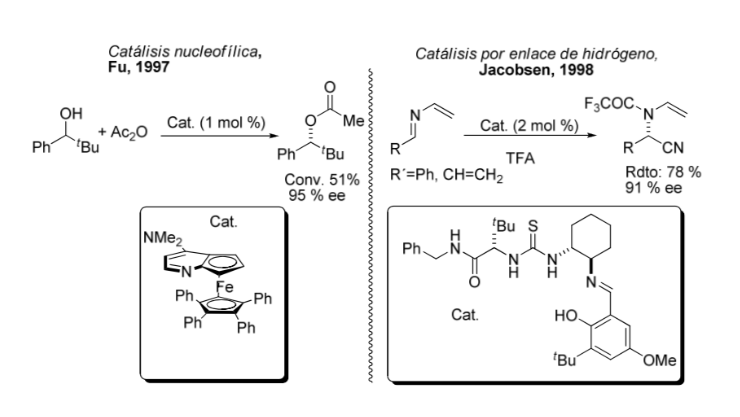
Comparison between organometallic and asymmetric catalysis:
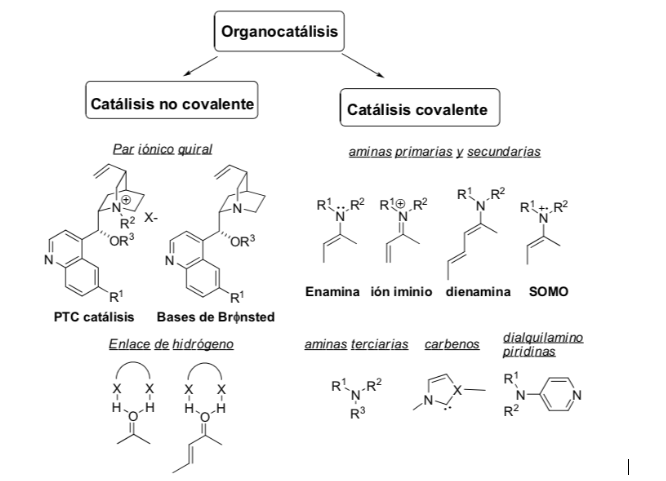
Activation method:
Sources to expand the search:
Berkessel, A. and Groeger, H. Asymmetric organocatalysis: from biomimetic concepts to applications in asymmetric synthesis” , Wiley-VCH: Weinheim, 2005.
Bui, T., Syed, S. and Barbas, CF, Thiourea-Catalyzed Highly Enantio- and Diastereoselective Additions of Oxindoles to Nitroolefins: Application to the Formal Synthesis of (+)-Physostigmine, J. Am. Chem. Soc . 131 , 8758-8759, 2009.
Hernández-Rodríguez, M. and Juaristi, E., Structurally simple chiral thioureas as chiral solvating agents in the enantiodiscrimination of carboxylic acids, Tetrahedron 63 , 7673-7678, 2007.
Juaristi, E. Nobel Prize in Chemistry 2001: The importance of asymmetric synthesis, Educ. quím . 13 , 6-7, 2002. .
Juaristi, E. Asymmetric synthesis of valuable amino acids, in Mexican scientific and humanistic contributions in the 20th century , Paredes, O. and Estrada, S., Eds., Fondo de Cultura Económica: México, 2008, p. 440-446.
Juaristi, E. Enantioselective synthesis of â -amino Acids , Wiley-VCH: New York, 1997.
Juaristi, E. and Soloshonok, VA (Eds.) Second edition of enantiose- lective synthesis of â -amino acids , Wiley: New York, 2005. Liu, Y., Melgar, R. and Juaristi, E. Enantioselective amination of
á-phenyl á-cyanoacetate catalyzed by chiral amines incorporating the á-phenylethyl auxiliary, J. Org. Chem. 72 , 1522-1525, 2007.
MacMillan, DWC, The advent and development of organo-catalysis, Nature 455 , 304-308, 2008.
Marigo, M., Juhl, K., and Jorgensen, KA, Catalytic, highly enantioselective, direct amination of beta-ketoesters, Angew. Chem., Int. Ed. 42 , 1367-1369, 2003.
Mukherjee, S.; Yang, JW; Hoffman, S.; List, B., Asymmetric enamine catalysis, Chem. Rev. 107 , 5471-5569, 2007.
Olivares-Romero, JL and Juaristi, E., Synthesis of Two Novel Chiral Diamines Derived from ( S )-Proline and their Evaluation as Precursors of Diazaborolidines for the Catalytic Borane-Mediated Enantioselective Reduction of Prochiral Ketones, Tetrahedron 64, 9992- 9998, 2008.
Seayad, J. and List, B., Asymmetric organocatalysis, Org. Biomol. Chem. 3 , 719-724, 2005.
Simon,L. and Goodman,JL,MechanismofBINOL−Phosphoric Acid-Catalyzed Strecker Reaction of Benzyl Imines, J. Am. Chem. Soc. 131 , 4070-4077, 2009.
Tanaka, K., Mori, A. and Inoue, S., The cyclic dipeptide cyclo[( S )-phenylalanyl-( S )-histidyl] as a catalyst for asymmetric addition of hydrogen cyanide to aldehydes, J. Org. Chem. 55 , 181-185, 1990.









