WHAT IS ASYMMETRICAL SYNTHESIS?
The asymmetric synthesis or also called chiral or enantioselective synthesis, is that which introduces or favors a desired chirality. This branch of organic synthesis is of vital importance for the manufacture of medicines since these in the body through various metabolic mechanisms only work at a specific chirality of the enzymes present in the process.
BASIC CONCEPTS IN ASYMMETRICAL SYNTHESIS:
REGIOSELECTIVE PROCESS:
A reaction is called regioselective when one of the possible structural isomers occurs predominantly:
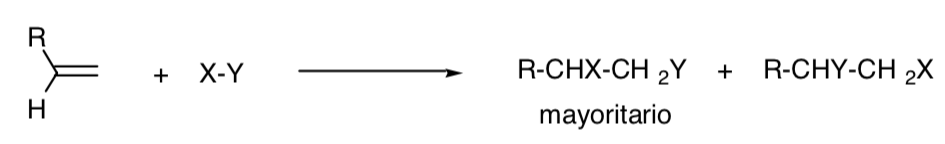
REGIOSPECIFIC PROCESS:
A reaction is called regiospecific when only one of the possible structural isomers is formed:
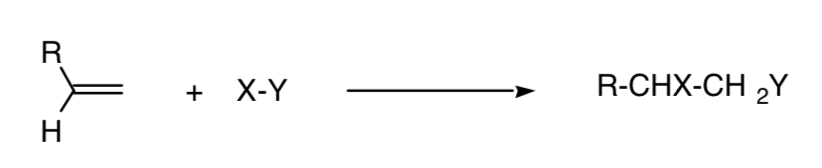
This concept can be extended to other processes, such as 4+2 cycloaddition processes:
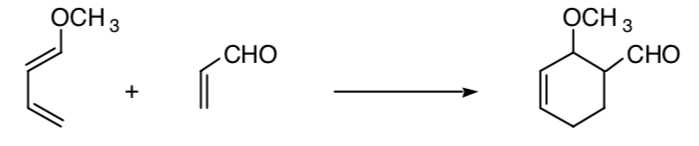
STEREOSELECTIVE SYNTHESIS:
A synthesis is called stereoselective when a stereoisomer of a certain structure originates in a considerably higher proportion than the rest of the other stereoisomers of the same structure.
We speak of an enantioselective process when one of the enantiomers is in the majority and of a diastereoselective process when a diastereomer or diastereomeric racemate is formed in the majority.
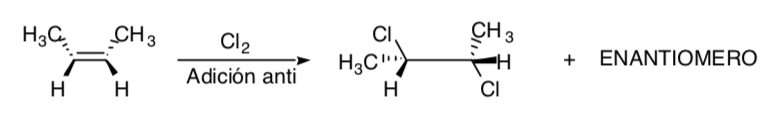
STEREOSPECIFIC SYNTHESIS:
It is one in which different stereoisomers of the starting substrate give rise to stereoisomerically different products:
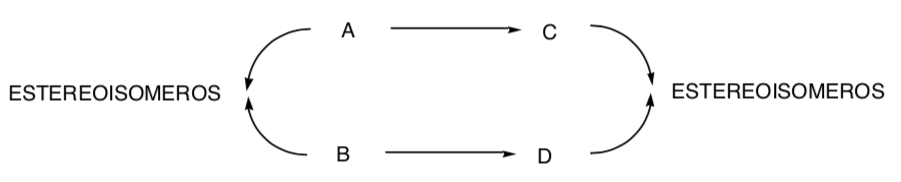
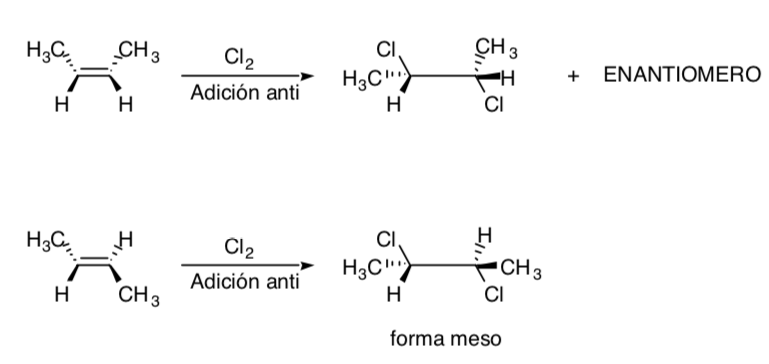
The chlorination of alkenes is an example of a stereospecific process:
STEREOSELECTIVE CONTROL OF A SYNTHESIS :
The need for stereoselective control in the creation of new chirality centers is evident in the successive reduction of one of the carbonyl groups and the double bond present in the Wieland-Miescher ketone. If the process is not stereoselective, four stereoisomers of the final product will be obtained:
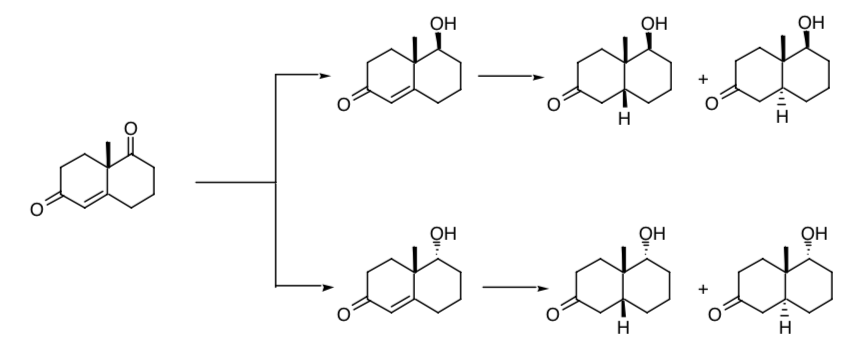
If we start from the racemate of said ketone we will obtain, from the reduction process, eight possible stereoisomers.
STEREOCHEMICAL CONSIDERATIONS:
A simplification of the stereochemical problem may consist of eliminating all the chirality centers and planning a synthesis in the most stereoselective way possible, for which not only the configuration of each chiral center must be taken into account, but also the influence of some chiral centers on others. If this influence does not exist (because they are distant), the problem can be simplified starting from structural units that include the chiral centers with the appropriate configuration.
Sometimes it is possible to use remote functional groups as control synthons, as is the case of the Simmons-Smith cyclopropanation in which a hydroxyl group allows the cyclopropanation to be carried out stereoselectively by coordination of the hydroxy group with the carbenoid species:
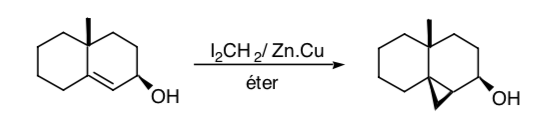
SYMMETRY CONSIDERATIONS:
The recognition of a symmetry in the molecule can be very useful, as is the case of squalene:
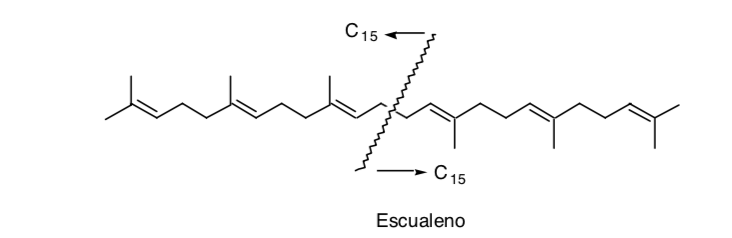
Although in reality its synthesis was not carried out by dimerization (C15 + C15) but by adding two C11 units to a central C8 unit (C11 + C8 + C11).
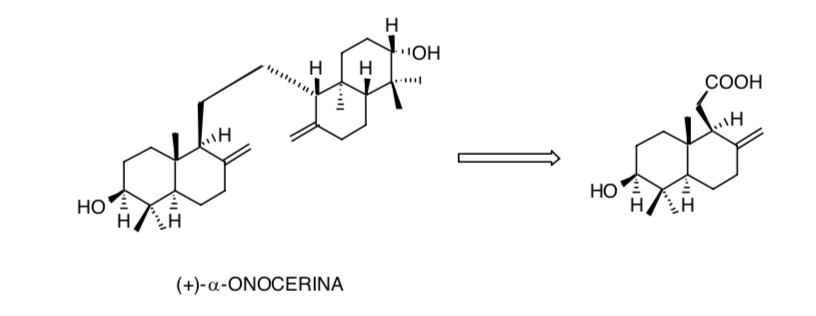
Consideration of symmetry in the (+)-α-onocerin molecule reduces the problem of creating eight chiral centers to only four:
Recommended sources for further information:
1) Guo-Qiang Lin, Yue-Ming Li, Albert SC Chan. Principles and Applications of Asymmetric Synthesis. Ed. Wiley-Interscience. Great Britain, 2001.
2) Mark Rizzacasa and Michael Perkins. Stoichiometric Asymmetric Synthesis. Ed. Sheffield Academic Press. USA and Canada. 2000.
3) Jonathan MJ Williams. Catalysis in Asymmetric Synthesis. Ed. Sheffield Academic Press. USA and Canada. 1999.
4) RA Aitken and SN Kulényi. Asymmetric Synthesis. Ed. Blackie Academic and Professional. Great Britain, 1992.
5) Grossman RB The Art of Writing Reasonable Organic Reaction Mechanisms. Springer, New York. 2003
6) Norman ROC; Coxon JM Principles of Organic Synthesis. CRC Press, Boca Raton. 1993