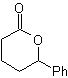
Baeyer–Villiger oxidation
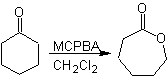
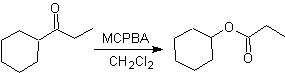
Another reaction that can be associated with the strategy of the Retrosynthesis is the oxidation of ketones by peroxyacids, better known as the Baeyer-Villiger reaction. In cyclic ketones, oxidation with peracids generates lactones. The groups attached to the asymmetric ketones have a migratory ability, which allows, in literal terms, to "insert an oxygen atom" between the carbonyl group and the migrating group, thus producing an ester or a lactone.
It should be taken into account that enones (α, β unsaturated ketones) are not good substrates for the Baeyer-Villiger oxidation, because the alkene is much more reactive than the ketone. However There are special structures where the alkene can be protected by a nearby substituent due to the steric effect and thus direct the attack of the peracid towards the carbonyl group.
| … |
|
Remember that the migratory aptitude of the different groups, in the Baeyer-Villiger reaction, is as follows:
H> Ph> 3º alkyl> cycloalkyl> 2º alkyl> 1º alkyl> Me
Propose a synthesis plan for the following molecules:
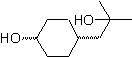
MOb 56
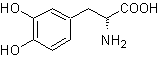
L-Dopa | . | MOb 57
| . | MOb 58
|
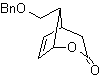
MOb 59
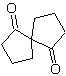
| MOb 60
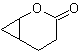
| mob 61
|
MOb 56. Retrosynthetic analysis.
The alpha amino acid
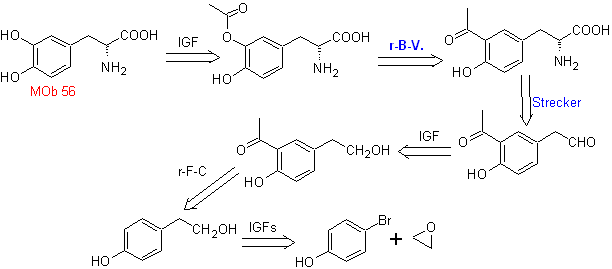
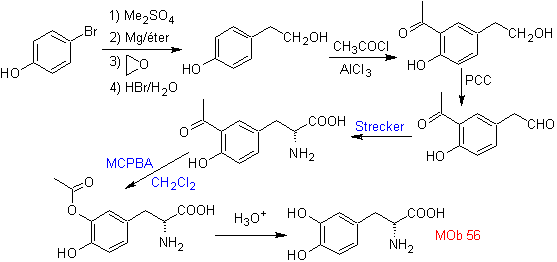
Synthesis. For For the formation of the required Grignard, the ortho OH of the benzene is protected. The Strecker synthesis allows the formation of the alpha amino acid, which is oxidized according to Baeyer-Villiger with a peracid and the product undergoes acid hydrolysis of the ester group, which leads to the formation of
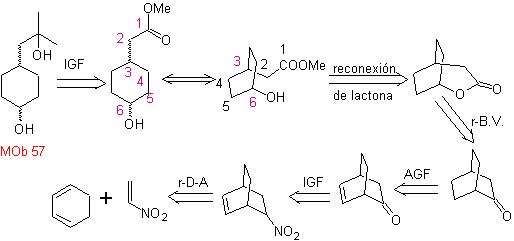
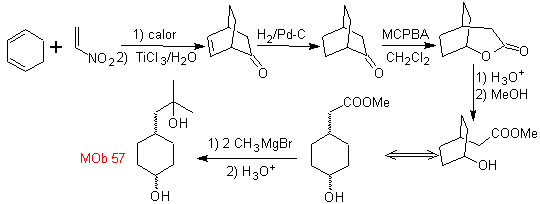
synthesis . It starts from the Diels-Alder reaction of a cyclohexadiene and a nitroethylene. The adduct formed is saturated, to proceed to the Baeyer-Villiger oxidation. The opening of the lactone and esterification of the acid group, forms an intermediate that is then treated with an excess of methyl magnesium bromide, to obtain
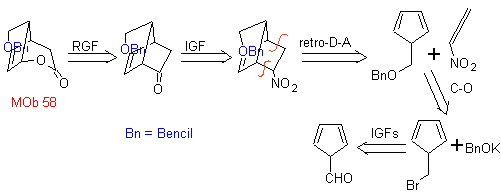
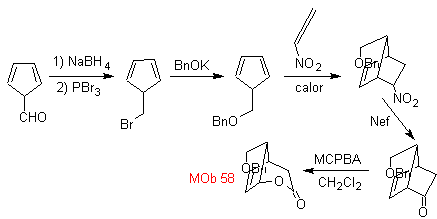
MOb 58. Retrosynthetic analysis. The double bond conjugated to a C=O group, present in
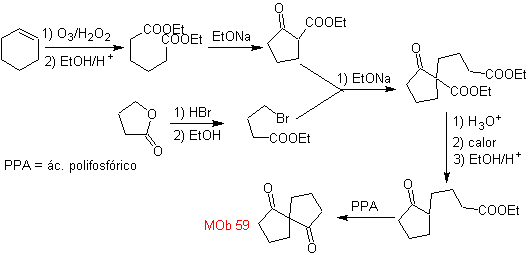
MOb 59 . Retrosynthetic analysis. It proceeds to disconnect
Once again, an intermediate or precursor 1,3-diCO is generated, which, when disconnected, generates a new 1,6-diCO structure, which can now be reconnected to reach cyclohexene as starting material.
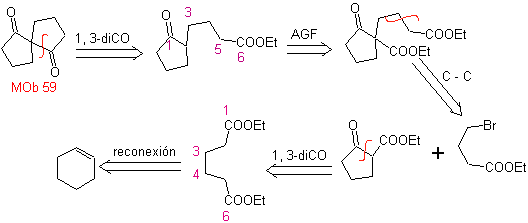
synthesis . The oxidative opening of a cyclohexene allows obtaining the precursor molecule that, after reacting with the γ-bromoester, gives rise to a molecule that, after a Dieckmann reaction, is easily transformed into
MOb 60 . Retrosynthetic analysis. It is assumed that the formation of
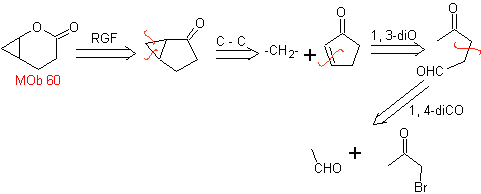
synthesis . The acetaldehyde enolate combines with α-bromoketone to form a 1,4-diCO molecule, which in a basic medium and EtOH condenses with dehydration to form an α,β-unsaturated cyclopentanone. The Simonns-Schmidt reaction is continued to form cyclopropane and a subsequent oxidation of it according to Baeyer-Villiger produces
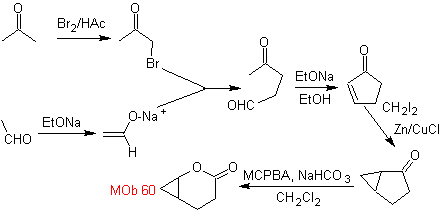
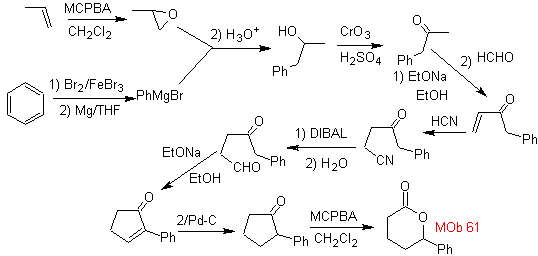
MOb 61 . Retrosynthetic analysis . It is assumed that
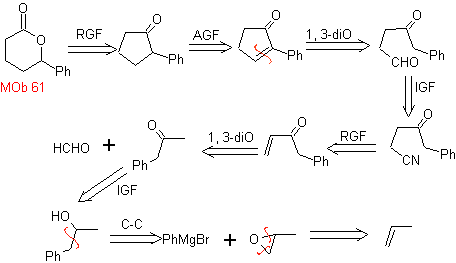
Synthesis. Propene and benzene are taken as starting materials, the strategy is to reduce the nitrile to CHO with DIBAL in hexane, to form the intermediate that by Robinson annulation (or annulation) and subsequent saturation, provides the adequate cyclopentanone to be oxidized by the Baeyer-Villiger procedure, to form