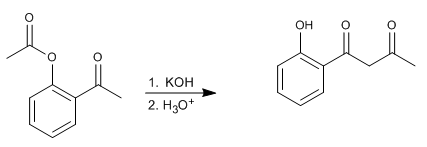
The Baker-Venkataraman rearrangement transforms aromatic ortho-acyloxyketones to beta-diketones by basic treatment (catalysis). Beta-diketones are of great interest in the synthesis of chromones, flavones and coumarins. The most commonly used bases in the reaction are: KOH, potassium tert-butoxide, sodium in toluene, potassium hydride.
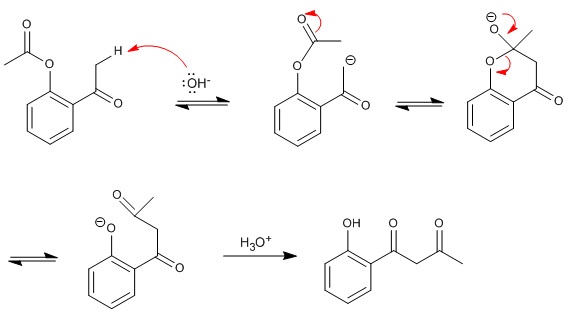
The reaction mechanism begins with the subtraction of alpha hydrogen from the ketone, forming an enolate that attacks the carbonyl of the acyloxy group, giving rise to the formation of a cyclic intermediate that yields the beta diketone after final cleavage.