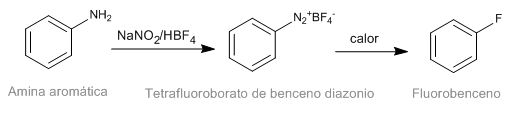
The Schiemann reaction consists of the thermal decomposition of aromatic diazonium tetrafluoroborates to yield the corresponding fluorinated derivative. Although diazonium salts are intestable, diazonium tetrafluoroborates have significant stability and can be prepared in good yield. Diazonium tetrafluoroborate is prepared from aromatic amines by the diazotization reaction.
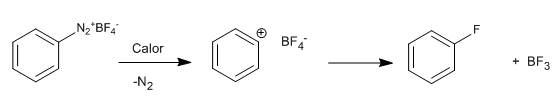
The reaction mechanism most likely involves a positively charged intermediate, resulting from the decomposition of the diazonium salt, which is attacked by fluorides from the tetrafluoroborate.