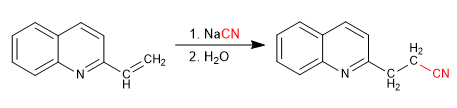
Pyridines with 2,4-position alkyl groups have acidic hydrogens on the carbon adjacent to the pyridine ring.
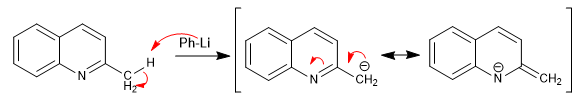
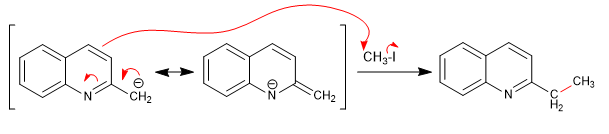
Hydrogens can be subtracted using strong bases. The base formed is a good nucleophile and allows the attack of various carbon electrophiles.
Isoquinoline has acidic hydrogens in position 1.
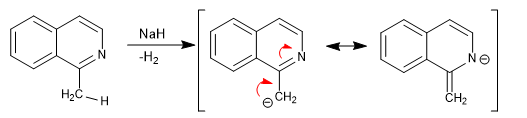
The delocalization of the charge towards the nitrogen atom is key for the hydrogen to present acidity. The 3-position hydrogens (in isoquinoline) are much less acidic since such delocalization causes the breakdown of aromaticity in the carbocycle.
Quinolines with 2,4-vinyl groups or isoquinolines with 1-vinyl groups are attacked by nucleophiles on the vinyl double bond.