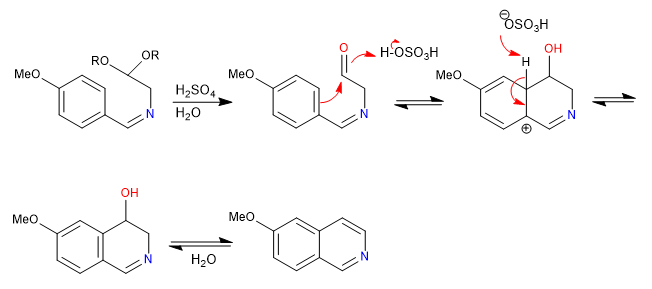
The Pomeranz-Fritsch synthesis prepares isoquinolines by reacting benzaldehydes with protected a-aminoaldehydes in the form of acetals. In a first stage, the imine is formed by reaction between benzaldehyde and the amine. In a second step, the acetal is broken with aqueous sulfuric acid, leaving the aldehyde free, which cyclizes under benzene attack. 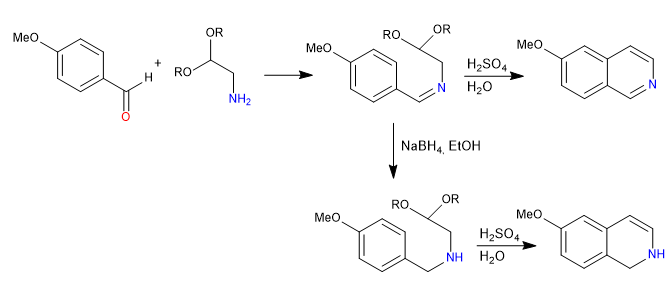
Mechanism: