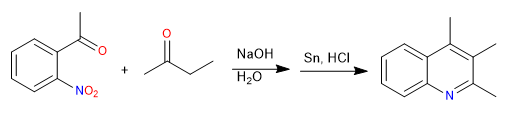
The Friedländer synthesis prepares quinolines from an ortho-acetylated nitrobenzene and a ketone. The synthesis begins with an aldol condensation in a basic medium. Reduction of the nitro group to amino allows cyclization by imine formation.
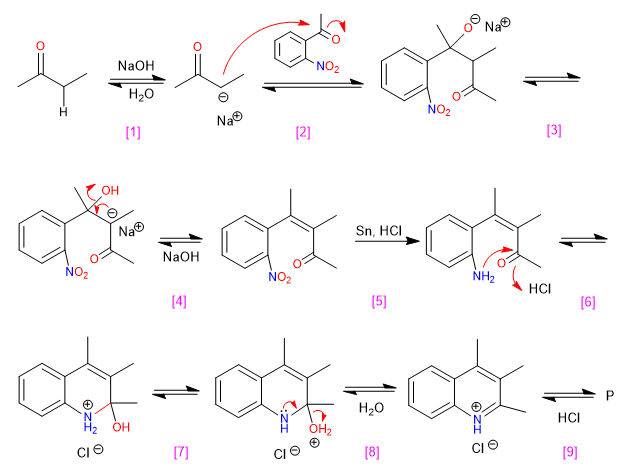
Mechanism:
This synthesis can also be carried out by direct reaction of o-aminoacetophenone and an enolizable carbonyl in a basic medium.
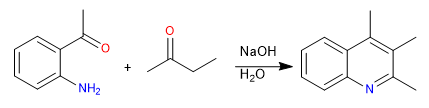
The mechanism can occur in two ways:
1. Formation of the imine, tautomerism to enamine and attack on the carbonyl
2. Aldol condensation and subsequent formation of the imine.