Of the three heterocycles studied in this topic, pyrrole is the most reactive in electrophilic substitution. In acid media it polymerizes, nitra in the presence of nitric acid and acetic anhydride, sulfone with the pyridine-SO 3 complex, halogen in the presence of dilute halogen solutions at low temperature and gives the Vilsmeier and Mannich reactions.
pyrrole polymerization
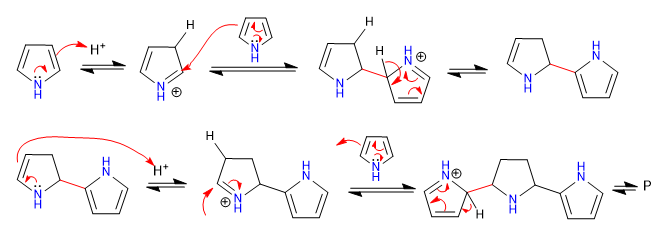
Pyrrole polymerizes easily in acid media, even the presence of light is sufficient to initiate said polymerization. This is the first example of electrophilic aromatic substitution of this heterocycle.
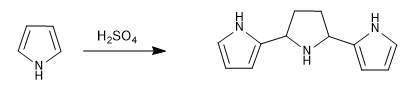
Mechanism: