Synthesis of 1,3 and 1,5-dicarbonyl compounds
Propose a synthesis design by the disconnection method (synthon method) from simple and affordable materials, for the following molecules:
(Remember that if it is not possible to propose a direct disconnection, it will be necessary to resort to the strategy of previously functionalizing the MOb, until reaching an applicable disconnection model)
SOLUTIONS TO PROPOSED PROBLEMS
MOb 01 does not have any dioxygen ratio, an aspect that in some sense is an advantage for the chemist. That is, the strategy to be used will allow the search for dioxygenated relationships in the precursor molecule (synthetic equivalent) with a certain degree of freedom, that is, it can be postulated, depending on the structure of the MOb, a range of dicarbonyl and/or hydroxycarbonyl relationships, in positions relative 1.2, 1.3 , 1.4, 1.5 and/or 1.6 or their variants, such as α, β-unsaturated carbonyl compounds.
On this occasion, the synthesis will be exercised, resorting to the 1.3 and/or 1.5 dioxygenated ratios. In this way, the target molecule and precursors will be converted into disconnectable structures according to a known and pre-established pattern.
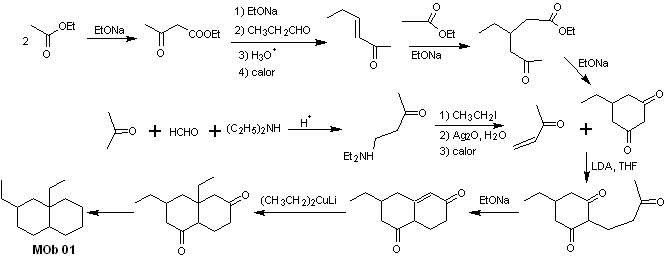
Solution MOb 01
Retrosynthetic analysis : In the molecule in question, one can start with perform an AGF, placing a C=O group in the structure of the precursor molecule, in such a position, which allows later to make another AGF, with a double bond located on the alpha and beta carbon with respect to the carbonyl, to proceed to disconnect it.
The α, β unsaturation, with respect to C=O, must be sought as the most substituted alkene of the alternatives that could exist. The presence of a substituent in the beta position at C=O leads us to think that it could have added as a nucleophile to an α, β unsaturated carbonyl compound, according to the Michael conjugate addition reaction. Based on these considerations, the following retrosynthetic analysis can be postulated for MOb 01:
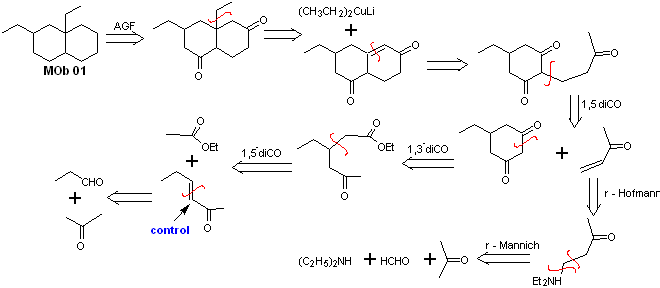
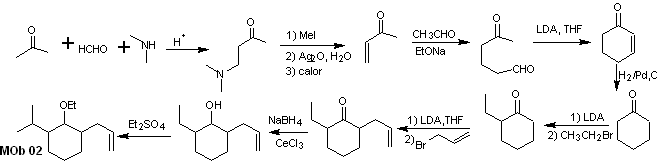
Synthesis of MOb 01 : The condensation between an enolizable aldehyde and ketone normally produces self-condensation or cross-condensation products. This can be avoided, resorting to the strategy of exerting control, in the nucleophile of the ketone, as can be seen in the attached scheme.
On the other hand, when it is required to use vinyl ketones as a substrate in the Michael reaction, it would mean the use of formaldehyde. Unfortunately, this aldehyde, being very reactive in a basic medium, tends to cause polymerization reactions, which drastically lower the yield of the synthesis. For this reason, the Mannich reaction and the Hofmann elimination are adequately combined for vinyl ketones with high yields. .
The Hofmann elimination could be carried out in the same basic medium used for the Michael reaction, so it is not necessary to isolate the vinyl ketone.
However, due to the still limited experience in synthesis, the use of silver oxide in an aqueous medium will be postulated to achieve the elimination of the amine and formation of the vinyl ketone.
Solution MOb 02
Retrosynthetic analysis : The presence of the ethyl and propenyl groups in position 2 to the ether group guides the disconnection strategy by functionalizing the ether to the ketone, and subsequently dealing with the alkylation of a cyclohexanone enolate. The disconnections that occur in the precursor molecules have been explained in the solution of MOb 01.
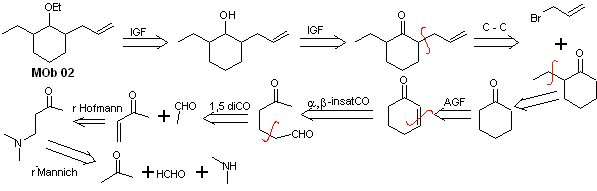
Synthesis. To prepare the kinetic enolate, LDA will be used, because this weakly nucleophilic base has a high steric hindrance and abstracts a proton from the less hindered carbon of the ketone. On the other hand, to guarantee the reduction of the carbonyl group to alcohol in the presence of an alkene group, NaBH 4 will be used in the presence of Ce +3 salts.
Solution MOb 03
Retrosynthetic analysis : The relative position in 1,5 of the hydroxyl groups, allows proposing a 1,5-diCO precursor molecule, which is disconnected according to this model by the bond that joins the cyclohexenone to the rest of the molecule, to form two molecules precursors α,β-unsaturated CO.
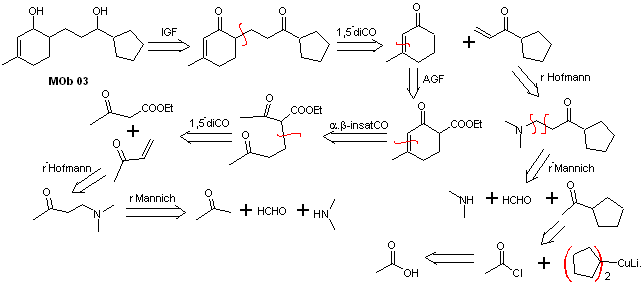
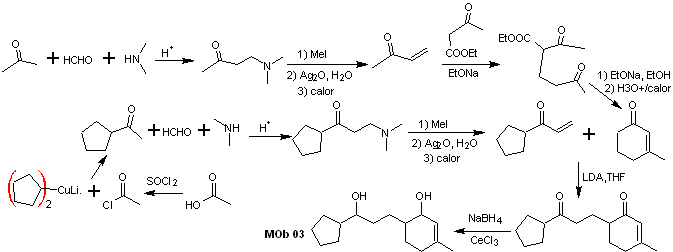
Synthesis. It is necessary to exercise control for the formation of vinyl ketones, for which the Mannich reaction is adequately combined, followed by the Hofmann elimination. For the Michael reaction, a ketoester formed by the Claisen condensation of the ethyl acetate ester can be used as a nucleophile, thus avoiding the self-condensation of the ketone.
Solution MOb 04
Retrosynthetic analysis : The OH in position 1,3, can be transformed to 1,3-diCO, to proceed to the disconnection according to this model, which originates two synthetic equivalents, of which it is subjected to functionalization by adding –COOEt and a point of unsaturation in 3,5-diphenylcyclohexanone. The generated precursors present dioxygenated models for their respective disconnection.
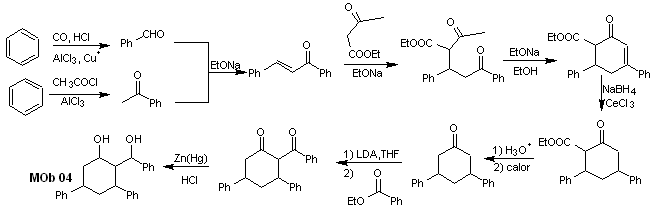
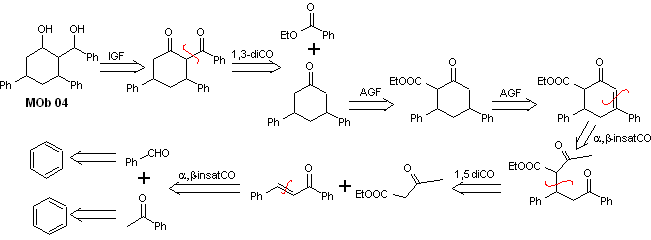 Synthesis. It starts from benzene and controls the Michael reaction and condensations.
Synthesis. It starts from benzene and controls the Michael reaction and condensations.
Solution MOb 05
Retrosynthetic analysis : Various dicarbonyl relationships can be considered for the structure of the precursor molecule (synthetic equivalent) of MOb 05. For example, by locating the first C=O group attached to benzene, a 1,3-diCO relationship can be postulated, an α , β-insatCO or 1,5-diCO, involving cyclohexane, with one or two C=O groups. This last option is assumed, since there is an additional methyl group between the C=O groups, which is easy to prepare, precisely due to the activating effect of the two C=O groups, on the nucleophile that is formed in basic medium. Based on these considerations, the following retrosynthetic analysis scheme for MOb 05 can be postulated.
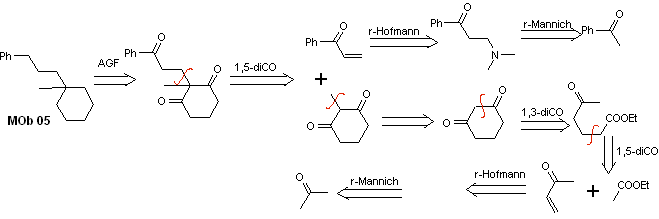 synthesis . Vinyl acetophenone is prepared in good yields through the Mannich reaction, followed by Hofmann elimination. On the other hand the 1,3-cyclohexanedione, is prepared from the Claisen condensation of a ketoester in the 1,5-diCO position. This diketone is alkylated and an H is obtained in a basic medium to form the nucleophile that will act on the a,b-insatCO compound. The C=O groups of the tricarbonyl molecule formed are reduced to methyl by Clemmensen reduction, placing an excess of zinc amalgam in an acid medium.
synthesis . Vinyl acetophenone is prepared in good yields through the Mannich reaction, followed by Hofmann elimination. On the other hand the 1,3-cyclohexanedione, is prepared from the Claisen condensation of a ketoester in the 1,5-diCO position. This diketone is alkylated and an H is obtained in a basic medium to form the nucleophile that will act on the a,b-insatCO compound. The C=O groups of the tricarbonyl molecule formed are reduced to methyl by Clemmensen reduction, placing an excess of zinc amalgam in an acid medium.
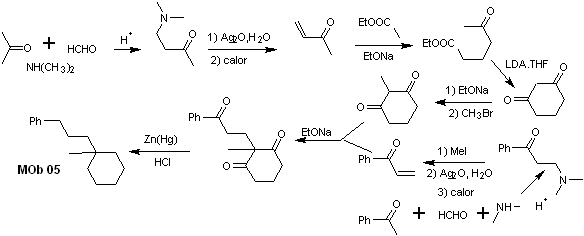
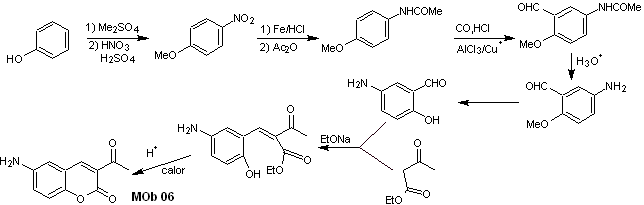
Solution MOb 06
Retrosynthetic analysis. It is disconnected by the lactone, an operation that allows us to glimpse an ester group. The precursor molecule formed, can also be subjected to the carbonyl compound, which could have been formed
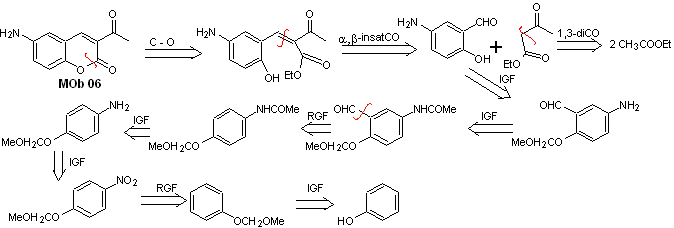
synthesis . The alcohol group is controlled by protecting it with dimethyl ether. Then the dicarbonyl compound must be synthesized
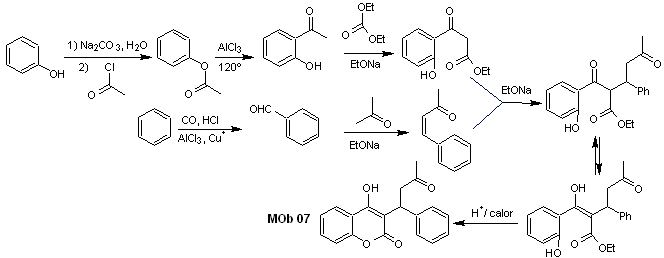
Solution MOb 07
Retrosynthetic analysis. Once again, the disconnection process begins due to the lactone function, the dioxygenated relationships that appear in dance clothes. It continues with the 1,5-diCO disconnection, and thus, it arrives at phenol and benzaldehyde.
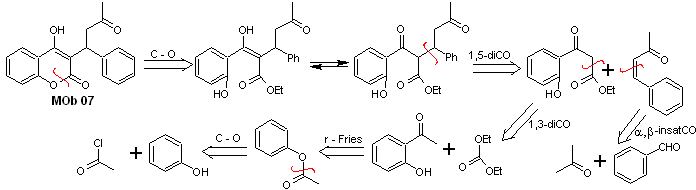
Synthesis. The starting materials are benzene and phenol, the required acetylphenol is prepared according to the Fries rearrangement reaction.
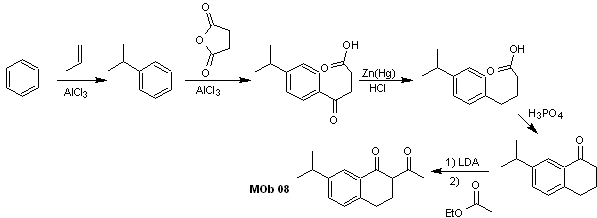
Solution MOb 08
Retrosynthetic analysis. The presence of the 1,3-diCO relationship in MOb 08 invites disconnection according to this disconnection model, the precursor molecule generated, it is not necessary to functionalize it to any disconnectable dioxygenated model, but rather the cyclization reactions of the acylation are used intramolecular Friedel-Crafts.
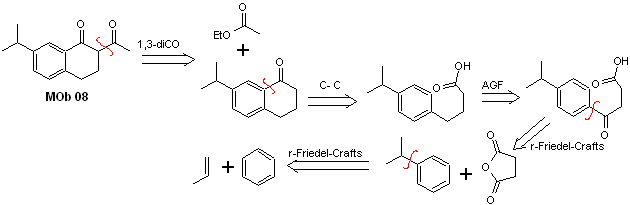
Synthesis. The acylation of cumene with succinic anhydride are the basic reactions that govern the synthesis. The orientation of the isopropyl group to a second electrophile that approaches the benzene ring is ideal to form the MOb.
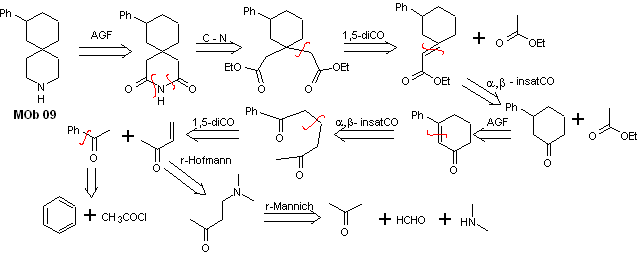
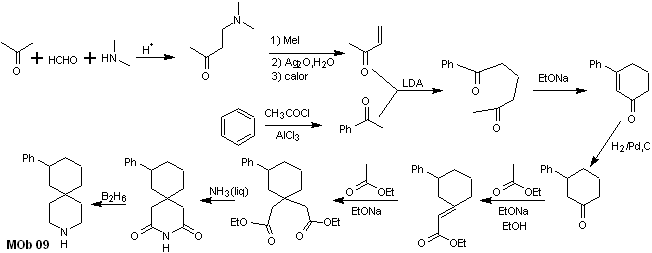
Solution MOb 09
Retrosynthetic analysis : The nitrogenous heterocycle of the MOb can be functionalized towards an easily disconnectable structure such as the amide function. The 1,5-diCO skeleton produced will be disconnected as predicted by this model, to arrive at an α, β -insatCO, which, when disconnected, generates a synthetic equivalent of a phenyl-substituted cyclohexanone. This last structure requires the proper use of retro-Hofmann and retro-Mannich, to arrive at simple and affordable starting materials.
Synthesis. The appropriate combination of the Mannich reaction and Hofmann elimination, followed by condensation reactions of the aldol and Michael type, dominate the synthesis strategy of MOb 09,
MOb 10 solution
Retrosynthetic analysis: The disconnection of the cyclic acetal leads to a precursor molecule, easily convertible into a disconnection model known as α , β -insatCO. The dioxygenated functions that are subsequently generated are disconnectable, by known models.
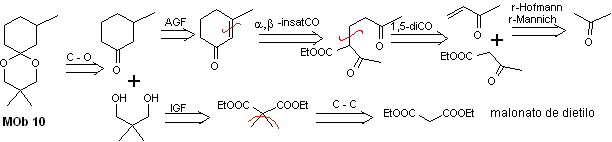
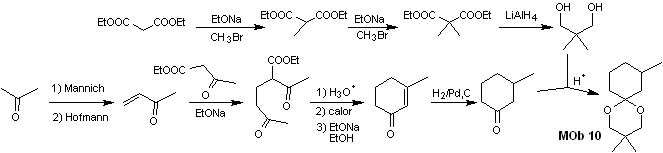
Synthesis. Diethyl malonate is the simple starting material required for the synthesis on the one hand and ethyl acetoacetate on the other. E diethyl malonate is doubly methylated and by reduction thereof with excess LiALH 4 , the diol is prepared. Which in an acidic medium will form the cyclic acetal.
Solution Mob 11
Retrosynthetic analysis. The most appropriate functionalization requires the addition of two installations conjugated with the C=O group, this strategy will be justified later, when it is requires a nucleophile to add to a Michael substrate.
On the other hand, it will be necessary to exercise control, in some condensations, to avoid the self-condensation of acetone, for which the acetoacetic ester is used, the result of the Claisen condensation of the ethyl acetate ester.
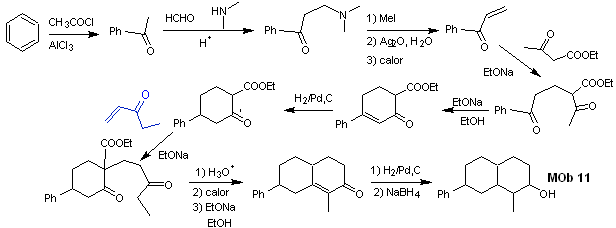
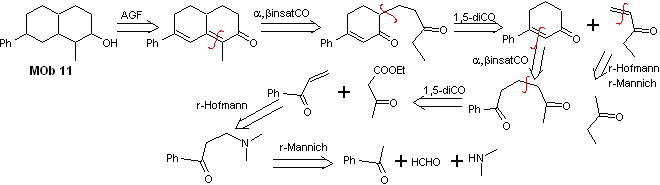 synthesis . The initial reactions of the synthesis have already been abundantly explained in the solution of the preceding MObs, it should only be noted that the intramolecular condensation reaction required is the Robinson annelation reaction.
synthesis . The initial reactions of the synthesis have already been abundantly explained in the solution of the preceding MObs, it should only be noted that the intramolecular condensation reaction required is the Robinson annelation reaction.
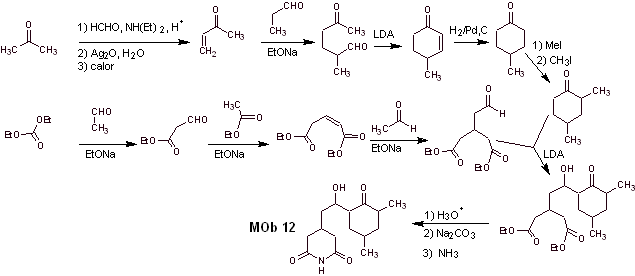
Mob 12 solution
Retrosynthetic analysis : The initial disconnection by the amide bonds generates a precursor molecule with various dioxygen ratios, from which 1,3-diO is selected, to divide the molecule into two important fragments and with each of them proceed to the necessary disconnections to generate a convergent synthesis design.
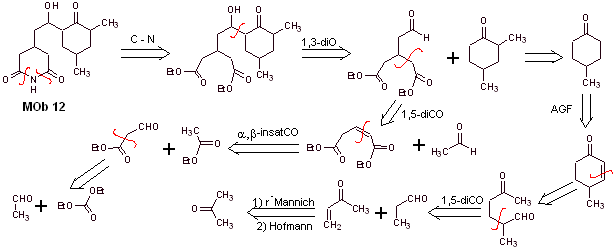
Synthesis. Proper handling of an aldehyde, generating more acidic hydrogens on its alpha carbon, is part of a good synthesis strategy.
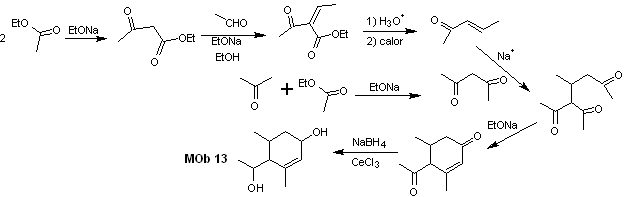
Solution MOb 13
Retrosynthetic analysis. The dihydroxy compound, MOb 13, is transformed into a dicarbonyl compound, to generate a precursor molecule, disconnectable according to the α, β – insat.CO model, to proceed with 1,5-diCO and others of this nature, until arriving at simple starting materials.
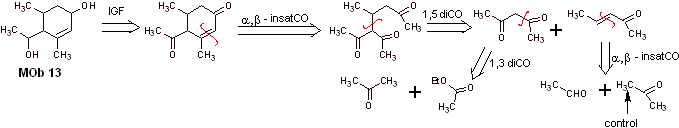 Synthesis. Control must be exercised in some base-catalyzed condensation reactions, to avoid the respective self-condensations, which will generate low synthesis yields.
Synthesis. Control must be exercised in some base-catalyzed condensation reactions, to avoid the respective self-condensations, which will generate low synthesis yields.
Solution MOb 14
Retrosynthetic analysis . This molecule has a resemblance to MOb 11, so the disconnections that are made will have some similarity.
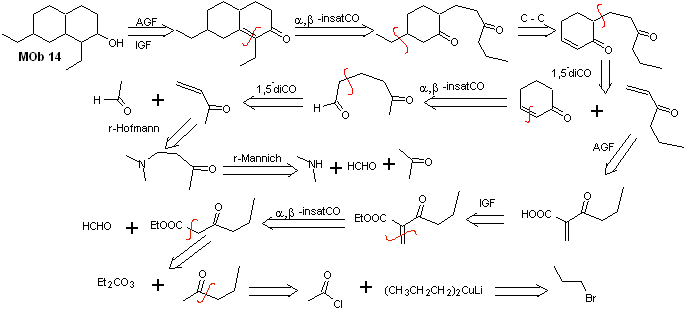 Synthesis
Synthesis
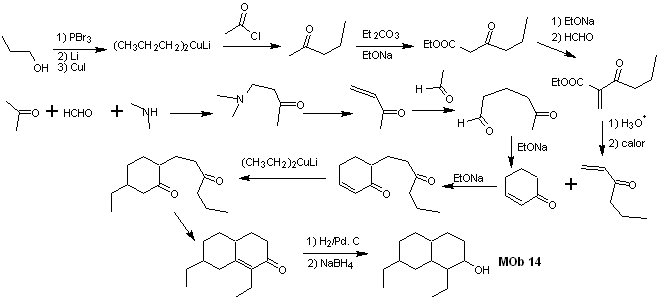
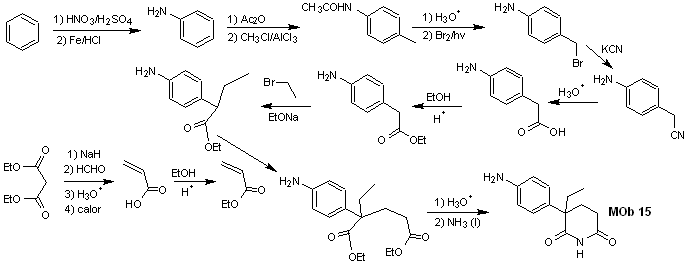
Solution MOb 15
Retrosynthetic analysis. The disconnection of the NO bonds of the lactam makes it possible to generate a precursor molecule, which can be disconnected according to the models already studied and to exercise control to avoid competition reactions.
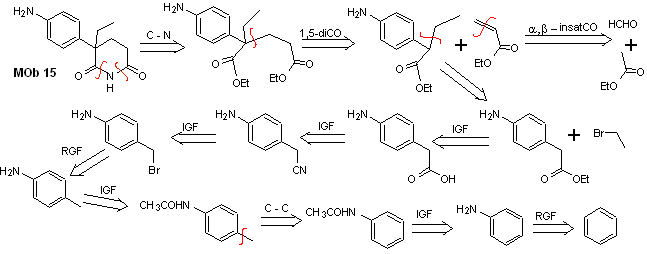 synthesis . Up to now, all the reactions have been repeatedly explained, so in this exercise, it will be taken for granted that the chemistry of dioxygenated compounds requires synthesis control measures to rectify it in light of the results obtained.
synthesis . Up to now, all the reactions have been repeatedly explained, so in this exercise, it will be taken for granted that the chemistry of dioxygenated compounds requires synthesis control measures to rectify it in light of the results obtained.