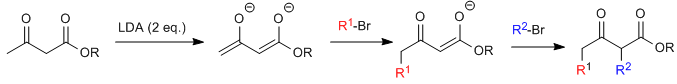
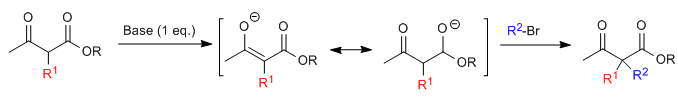
Acetylacetic synthesis allows ketones to be prepared by C-alkylation of ethyl acetylacetate (ethyl 3-oxobutanoate). Ethyl acetoacetate can be deprotonated at the C2 or C4 positions depending on the type and amounts of base used. The hydrogens at the C2 position they present significant acidity (pKa=11) due to stabilization of the conjugate base on the two neighboring carbonyls.In the presence of a base equivalent (alkoxides, LDA, NaHMDS, etc.) a ketoester enolate capable of attacking numerous carbonated electrophiles.
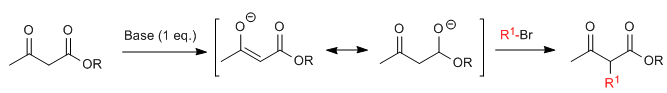
Where R 1 is a primary or secondary alkyl, allyl, or benzyl group.
The monoalkylated substrate is amenable to a second alkylation by adding a second equivalent of base followed by the electrophile.
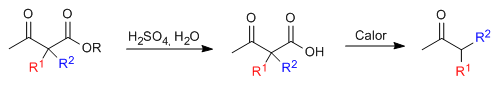
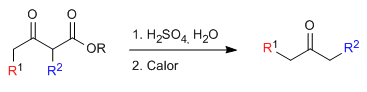
The monoalkylated or dialkylated ketoester is then subjected to an acid hydrolysis step which transforms it into a ketoacid sensitive to decarboxylation by heating.