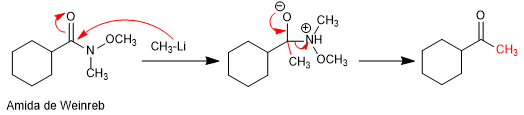
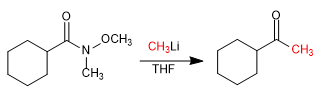
In 1981, Weinreb and Nahm obtained ketones by adding lithium or magnesium organometallics to N-methyl-N-methoxyamides.
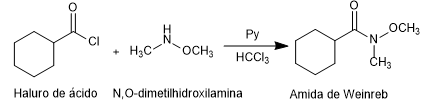
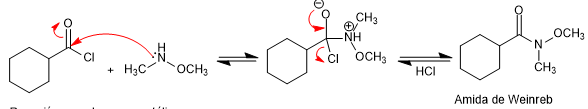
Weinreb's amides are prepared from acid halides or anhydrides by reaction with N,O-dimethylhydroxylamine in the presence of base (pyridine).
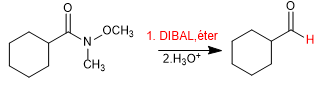
Weinreb's amide can be reduced with DIBAL yielding the corresponding aldehyde.
It is also possible to obtain Weinreb's amide from esters and lactones, catalyzing the reaction with a Lewis acid such as trimethylaluminium.
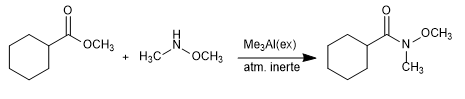
Mechanism:
Amide formation:
Reaction with organometallic: